- Tous
les articles sur la contagion du
bâillement
- All
articles about contagious
yawning
-
-
- Communication is an essential aspect of
animal social life. Animals may influence one
another and come together in schools, flocks,
and herds. Communication is also the way sexes
interact during courtship and how rivals settle
disputes without fighting. However, there are
some behavioral patterns for which it is
difficult to test the existence of a
communicatory function, because several types of
sensory modalities are likely involved. For
example, contagious yawning is a communicatory
act in mammals that potentially occurs through
sight, hearing, smell, or a combination of these
senses depending on whether the animals are
familiar to one another. Therefore, to test
hypotheses about the possible communicatory role
of such behaviors, a suitable method is
necessary to identify the participating sensory
modalities. The method proposed here aims to
obtain yawn contagion curves for familiar and
unfamiliar rats and evaluate the relative
participation of visual and olfactory sensory
modalities. The method uses inexpensive
materials, and with some minor changes, it can
also be used with other rodent species such as
mice. Overall, the method involves the
substitution of clear dividers (with or without
holes) with opaque dividers (with or without
holes) that either allow or prevent
communication between rats placed in adjacent
cages with holes in adjoining sides.
Accordingly, four conditions can be tested:
olfactory communication, visual communication,
both visual and olfactory communication, and
neither visual nor olfactory communication. As
social interaction occurs between the rats,
these test conditions simulate what may occur in
a natural environment. In this respect, the
method proposed here is more effective than
traditional methods that rely on video
presentations whose biological validity can
raise concerns. Nonetheless, it does not
discriminate between the potential role of
hearing and roles of smell and vision in yawn
contagion.
-
-
La communication est un aspect essentiel de la
vie sociale des animaux. Les animaux peuvent
s'influencer et se réunir en troupeaux ou
autres groupes sociaux. La communication est
également le moyen par lequel les sexes
interagissent pendant les parades et par
laquelle les rivaux règlent leurs
différends sans se battre. Cependant, il
existe des schémas comportementaux pour
lesquels il est difficile de tester l'existence
d'une fonction de communication, car plusieurs
types de modalités sensorielles sont
probablement impliqués. Par exemple, le
bâillement contagieux est un acte de
communication chez les mammifères qui se
produit potentiellement par la vue, l'ouïe,
l'odorat ou une combinaison de ces sens, selon
que les animaux se connaissent ou non. Par
conséquent, pour tester des
hypothèses sur le rôle communicatif
possible de tels comportements, une
méthode appropriée est
nécessaire pour identifier les
modalités sensorielles participantes. La
méthode proposée ici vise à
obtenir des courbes de contagion de
bâillement pour des rats qui se
connaissent et ou pas et à évaluer
la participation relative des modalités
sensorielles visuelles et olfactives.
-
- La méthode utilise des moyens peu
coûteux, et avec quelques modifications
mineures, elle peut également être
utilisée avec d'autres espèces de
rongeurs telles que les souris. Globalement, la
méthode implique la substitution de
séparateurs clairs (avec ou sans trous)
par des séparateurs opaques (avec ou sans
trous) qui permettent ou empêchent la
communication entre les rats placés dans
des cages adjacentes avec des trous dans les
côtés adjacents. En
conséquence, quatre conditions peuvent
être testées : la communication
olfactive, la communication visuelle, la
communication visuelle et olfactive, et la
communication ni visuelle ni olfactive. Comme
les interactions sociales se produisent entre
les rats, ces conditions de test simulent ce qui
peut se produire dans un environnement naturel.
À cet égard, la méthode
proposée ici est plus efficace que les
méthodes traditionnelles qui reposent sur
des présentations vidéo dont la
validité biologique peut susciter des
critiques.
- Néanmoins, ce test ne fait pas de
distinction entre le rôle potentiel de
l'audition et celui de l'odorat et de la vision
dans la contagion des bâillements.
Introduction
- Traditionally, communicatory behavior has
been studied from two perspectives. From one
perspective, ethologists observe and record the
behavior of animals in natural settings and
attempt to recognize its adaptive value1. The
particular sense or senses involved have not
been the primary interest of these studies. From
another perspective, physiologists are more
interested in unraveling the mechanisms by which
animals communicate1; hence, laboratory studies
have provided methods to address the role that
sensory modalities play in communication2,3.
These two perspectives are indeed complementary,
because knowledge of both adaptive value and
immediate mechanisms is necessary to gain a
comprehensive understanding of communicatory
behaviors in the social life of animals.
-
- Yawning behavior is a conspicuous component
of the behavioral repertoire in several species
of vertebrates4, ranging from fish to primates5.
It can be described as a slow opening of the
mouth and maintenance of its open position,
followed by a more rapid closure of the
mouth5.
-
- The duration of the whole sequence depends
on the species; for example, primates yawn for
longer durations than non-primate species6. In
many species, with humans being the exception,
males tend to yawn more frequently than
females7. This feature might underpin the
possible communicatory function of yawning,
although regular patterns of yawning and its
daily frequency may also suggest a physiological
function. In rats, spontaneous yawning follows a
circadian rhythm, with peaks of high frequency
occurring in the morning and afternoon8,9.
-
- One interesting feature of yawning behavior
is that it can be a contagious act (when the
releasing stimulus of a behavior happens to be
another animal behaving in the same way10) in
several species of vertebrates11,12,13,14,15,16,
including birds17 and rodents18. Furthermore,
recent evidence has indicated that contagious
yawning may reflect a communicatory role,
because the yawning of one rat can affect the
physiological state of another when exposed to
olfactory cues19. However, whether or not
yawning has a communicatory role is still under
debate20,21, and analyzing contagious yawning is
an essential first step to solve this
issue.
-
- On the other hand, contagious yawning has
been linked to an animal's ability to empathize
with the perspectives of other animals; hence,
closely related individuals are more likely to
show contagion4. This hypothesis has been
frequently tested in laboratory conditions in
which animals are presented with yawn stimuli on
video12,13; hence, contagion can only occur
through visual cues. Other investigations have
assessed yawn contagion in more natural
conditions using groups of animals14,15. A major
problem of this is that socially interacting
animals often respond to cues and exchange
signals that are conveyed through combinations
of sensory modalities. Disentangling the actual
senses involved in a given behavior from their
combined effects is not always an easy task.
Typically, researchers pharmacologically or
surgically hinder an animal's use of a given
sense, then infer the role of that sense in the
relevant behavior2,3,18,22. Fortunately, there
are other methods in which only physical
barriers are used to either allow or impede
communication between animals23,24,25, thereby
achieving greater biological validity.
-
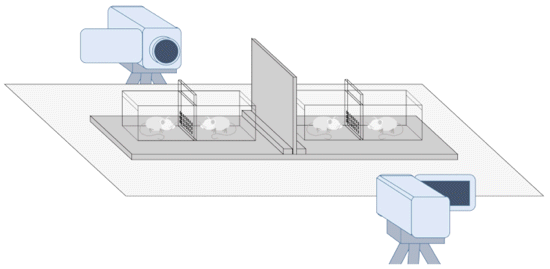
- The method proposed here has been
specifically designed to study contagious
yawning in familiar and unfamiliar rats in a
social setting. According to the empathetic
hypothesis, the former group should be more
susceptible to contagious yawning. The method
does not require the animals to be surgically or
pharmacologically deprived of any senses.
Instead, it works by placing the rats in
adjacent cages with holes and physically
obstructing their communication using either
clear or opaque dividers with or without holes.
Thus, four test conditions can be examined: (1)
olfactory communication (OC, perforated opaque
divider), (2) visual communication (VC,
nonperforated clear divider), (3) visual and
olfactory communication (VOC, perforated clear
divider), and (4) neither visual nor olfactory
communication (NVOC, nonperforated opaque
divider). Therefore, researchers can compare the
relative contributions of olfactory, visual, and
to some extent, auditory cues in yawn contagion.
This approach is not new, as similar methods
have been used to isolate the senses involved in
certain communicatory behaviors in animals such
as lizards23 and mice26. In fact, Gallup and
colleagues27 have used a similar method to
demonstrate the role of visual cues in
contagious yawning in budgerigars. The main
features of these methods are simulation of a
social context and the minimal stress inflicted
on the animals. Furthermore, the use of
interacting animals increases the biological
validity of the conclusions.
-
- There are several ways to measure contagious
yawning25,28. Dr. Stephen E. G. Lea (personal
communication, 2015) helped us numerically adapt
a method previously employed by
primatologists13,14 for an earlier analysis of
the data used here18. Presented in this protocol
is an enhanced version of this method with a
wider range of applications. It consists of
weighting the total number of a rat's yawns,
within and outside of a given time window, by
the proportion of observation time corresponding
to the yawns within and outside the time
window.
-
- For example, if it is assumed that rats A
and B are observed for 12 min, their yawning is
recorded to the nearest minute, and a 3 min time
window is set to measure contagious yawning.
Next, the following sequences of yawns for each
of those rats are considered: rat A
(0,0,0,1,0,0,2,0,0,0,2,1) and rat B
(0,1,1,0,1,1,0,0,0,0,0,3). It should be noted
that each number (0-3) corresponds to the number
of yawns scored at each min. For rat A, during
minutes 1, 10, and 11 (numbers in bold type),
rat B does not yawn within the preceding 3 min
(the chosen time window) or within that minute.
In those minutes, rat A yawns a total of 2
times. Therefore, the yawn rate of rat A without
any yawn stimulus (non-post-yawn yawn rate) is
2/3 (i.e., 0.67 yawns/min). In the remaining 9
min, rat B yawns at least one time in either the
same minute or the 3 previous minutes. Rat A
yawns a total of four times in those 9 min.
Therefore, the yawn rate of rat A in response to
a yawn stimulus (post-yawn yawn rate) is 4/9
(i.e., 0.44 yawns/min). The application of the
same procedure to rat B yields a non-post-yawn
yawn rate of 2/3 (i.e., 0.66) and post-yawn yawn
rate of 5/9 (0.55).
-
- On the other hand, if yawning is recorded to
the nearest decimal of a minute, yawn contagion
will result in an adjusted post-yawn time. For
example, if the following yawn times are
recorded over a 12 min observation period for
rats A and B: rat A (2.3, 5.1, 5.8, 10.4, 10.8,
11.1) and rat B (1.2, 2.4, 4.5, 5.1, 11.2, 11.6,
11.8). For rat A, the time periods over which
rat B does not yawn within the past 3 min range
from 0 to 1.2
- min and from 8.1 to 11.2 min (i.e., 3.1
min), which yields a total of 4.3 min of
non-post-yawn time. The number of times that rat
A yawns during those times is three (numbers in
bold type), so the non-post-yawn yawn rate is
3/4.3 (i.e., 0.69), while the post-yawn yawn
rate is 3/7.7 (i.e., 0.38; the denominator from
12-4.3 min). Similarly, for rat B, the time
periods over which rat A does not yawn within
the past 3 min range from 0 to 2.3 min and from
8.8 to 10.4 min, which yields a total of 3.9
min. The number of times rat B yawns within
those periods is one, so the non-post-yawn yawn
rate is 1/3.9 (i.e., 0.25). Accordingly, the
post-yawn yawn rate is 6/8.1 (i.e., 0.74).
-
- While a near-contemporaneous match in
behavior is an ideal criterion to demonstrate
the presence of a contagion, aspects such as the
constraints on what an individual attends to,
time of reaction to a stimulus, distribution of
the behavior over time (e.g., yawning may occur
in episodes), and time to acclimatize to the
experimental setting all give rise to species
differences, making it difficult to use a unique
time window. This may be the reason why
researchers have used time windows that vary
from seconds5 to several minutes11, which
creates problems when comparing results28.
Because of this, it is proposed to repeat the
procedure described above for a range of time
windows to obtain yawn contagion curves and
compare the yawn contagion curves between
species.
-
- Equivalent yawn contagion curves can be
compared by randomly distributing the number of
yawns observed for each rat over the observation
period. Thus, the proposed method to measure
yawn contagion offers two types of controls: the
(1) yawn rate occurring outside of the time
window (non-post-yawn time) and (2) artificial
yawn contagion curve obtained from the random
distribution of the number of yawns. Therefore,
this approach to analyze yawn contagion is a
step forward from other procedures, such as
those comparing the percentage or frequency of
yawning within a single time window to that
occurring outside this window25, without taking
into account the actual times frames. The method
is complemented by an R-based program29 to
conveniently and objectively calculate the
probability of contagious yawning for one or
more time windows.
- To illustrate the usefulness of this method
and advantages of the R-based program, a data
set from a previously published study18 is used.
The experimental condition consisted of 144 male
rats allocated to either a familiar or
unfamiliar condition. The rats in each
experimental condition were subdivided into four
subgroups of nine pairs and exposed to any of
the four test situations described above. The
yawning behaviors of the rats in each
experimental condition and test situation were
then recorded over a period of 60 min.
-
-
- Discussion
- There are critical steps in the method that
should be taken into account to obtain
successful results. Familiar rats must share
home cages for at least 1.5 months after weaning
and before running the experiments. However,
unfamiliar rats must live in separate home
cages. In both cases, the pairs of rats must
come from different litters but be as similar in
age as possible. Regarding the observation
cages, their holes should match those in the
dividers, because this is the only way to
guarantee olfactory contact between the rats.
The dividers, on the other hand, should be clear
enough to ensure visual contact or opaque enough
to ensure no visual contact. The wooden
partition between one pair of rats and the other
must be sufficient to prevent the rats on one
side from seeing those on the other side.
Another crucial aspect is the appropriate design
of the experiment. Whenever there is a suspicion
that a process may be biased, a random procedure
should be implemented30.
-
- The method should not present serious
problems to users. Making the holes in the glass
was the main technical problem faced, and
because
- of that, acrylic was used instead.
Nonetheless, glass may be used for making the
entire observation cages, provided professional
advice is obtained. It should be ensured that
the edges of the holes are filed to avoid glass
splinters that may injure the rats. However,
modifying the main method is not recommended
(e.g., making the holes larger). Additionally,
use of a combined group of males and females may
make it difficult to detect yawn contagion.
-
- The dividers used here may be insufficient
to prevent the rats from using auditory cues,
because rats are able to produce and perceive
sounds at frequencies at which the materials of
the observation cages and dividers likely did
not block. However, this situation itself makes
it possible to infer that auditory cues caused
yawn contagion18 and that olfactory cues only
facilitated the recognition of the partner's
degree of familiarity. Therefore, the method
proposed here still provides reasonable evidence
to identify the senses involved in contagious
yawning and their intensities.
-
- Earlier methods have been designed to study
contagious yawning in laboratory conditions
mainly by presenting videos to the experimental
individuals12,13, but these were questionable
approaches in terms of biological validity. The
method presented here solves this concern by
using socially interacting animals in conditions
more similar to what occurs in the real world.
Furthermore, it is possible to simultaneously
explore the participation of several sensory
modalities in a single experimental set-up. It
is recognized that this method does not
absolutely discriminate between the effects of
auditory cues and other sensory cues. However, a
well-designed further experiment may allow
researchers to infer the most likely sensory
modality involved18. One possible noninvasive
solution is the use of white noise to mask
sounds and eliminate auditory cues. Similarly,
researchers may expose nai_ve rats to bedding
from OC rats to determine the role of olfactory
cues, which is a proven procedure in social
facilitation studies32.
- The use of this method can be extended to
study yawn contagion in other species. For
example, this set-up can be used after simple
modifications with animals such as mice and
hamsters to compare yawn contagion curves.
Comparisons among different species may reveal
unexpected patterns. The basic experimental plan
can work with larger animals such as guinea
pigs, cats, and rabbits. Likewise, the method
can be used to study other potentially
contagious behaviors such as grooming and
scratching. The R-based program can reduce the
time spent calculating yawn contagion for
several time windows and can be used to measure
yawn contagion in other vertebrate species,
provided the user has previously collected
relevant data.
-
- In summary, the main advantages of this
method are the leading to acquisition of yawn
contagion curves and aiding in discrimination
between the relative roles of sensory modalities
involved. The acquisition of yawn contagion
curves is, as far as we know, a novel approach
that may be useful to measure the strength of a
contagion and observe how this intensity varies
among species. Accordingly, the method can also
be used with some modifications in other animal
species such as sheep16, wolves15, dogs33,
snakes34, and fish5. In all these species except
snakes, yawning has been previously documented.
In fact, a method similar to the one presented
here has been used successfully in
budgerigars27. This method may also be used to
study other types of behavior that are
contagious. For example, behaviors such as
emotional reactivity, grooming, and scratching
in rodents may be contagious. In fact, the
method proposed here showed contagious emotional
reactivity in familiar rats18.
-
-
- References
- 1. Ce_zilly, F. A. History of Behavioural
Ecology. In Behavioural Ecology. Danchin, E.,
Giraldeau, L-A., Ce_zilly, F. Oxford University
Press. Oxford. 3-27 (2008).
- 2. Medina, L. M., Garcia, C. M., Urbina, A.
F., Manjarrez, J., Moyaho, A. Female vibration
discourages male courtship behaviour in the
Amarillo fish (Girardinichthys multiradiatus).
Behavioural Processes. 100, 163-168 (2013).
- 3. Mekdara, P. J., Schwalbe, M. A.,
Coughlin, L. L., Tytell, E. D. The effects of
lateral line ablation and regeneration in
schooling giant danios. Journal of Experimental
Biology. 221 (8), 175166 (2018).
- 4. Walusinski, O. Contagious Yawning. In
Encyclopedia of Animal Cognition and Behavior.
Vonk, J., & Shackelford, T.K. Springer
Nature. Switzerland AG. 1-5 (2018).
- 5. Baenninger, R. Some comparative aspects
of yawning in Bettasplendens, Homosapiens,
Pantheraleo, and Papiosphinx. Journal of
Comparative Psychology. 101 (4), 349-354
(1987).
- 6. Gallup, A. C., Church, A. M., Pelegrino,
A.J. Yawn duration predicts brain weight and
cortical neuron number in mammals. Biology
Letters. 12, 20160545 (2016).
- 7. Baenninger, R. On yawning and its
functions. Psychonomic Bulletin and Review. 4
(2), 198-207 (1997).
- 8. Ani_as, J., Holmgren, B., Urba_-Holmgren,
R., Egui_bar, J. R. Circadian variation of
yawning behavior. Acta Neurobiologiae
Experimentalis . 44
- (4), 179-186 (1984).
- 9. Holmgren, B. et al. Food anticipatory
yawning rhythm in the rat. Acta Neurobiologiae
Experimentalis. 51 (3-4), 97-105 (1991).
- 10. Byrne, R. W. The evolution of
intelligence. In Behaviour and Evolution,.
Slater, P. J. B., Halliday, T. R. Cambridge
University Press. Cambridge. 223-265
(1994).
- 11. Provine, R. R. Yawning as a stereotyped
action pattern and releasing stimulus. Ethology.
72 (2), 109-122 (1986).
- 12. Provine, R. R. Faces as releasers of
contagious yawning: An approach to face
detection using normal human subjects. Bulletin
of the Psychonomic Society. 27 (3), 211-214
(1989).
- 13. Anderson, J. R., Myowa-Yamakoshi, M.,
Matsuzawa, T. Contagious yawning in chimpanzees.
Proceedings of the Royal Society B: Biological
Sciences. 271 (Suppl 6), S468-S470 (2004).
- 14. Palagi, E., Leone, A., Mancini, G.,
Ferrari, P. F. Contagious yawning in gelada
baboons as a possible expression of empathy.
Proceedings ofthe National Academy of Sciences.
106 (46), 19262-19267 (2009).
- 15. Romero, T., Ito, M., Saito, A.,
Hasegawa, T. Social modulation of contagious
yawning in wolves. PLoS One. 9 (8), e105963
(2014).
- 16. Yonezawa, T., Sato, K., Uchida, M.,
Matsuki, N., Yamazaki, A. Presence of contagious
yawning in sheep. Animal Science Journal. 88
(1),195-200 (2017).
- 17. Miller, M. L., Gallup, A. C., Vogel, A.
R., Vicario, S. M., Clark, A. B. Evidence for
contagious behaviors in budgerigars
(Melopsittacus undulatus): an observational
study of yawning and stretching. Behavioral
Process. 89 (3), 264-270 (2012).
- 18. Moyaho, A., Rivas-Zamudio, X., Ugarte,
A., Egui_bar, J. R., Valencia, J. Smell
facilitates auditory contagious yawning in
stranger rats. Animal Cognition. 18 (1), 279-290
(2015).
- 19. Moyaho, A., Flores Urbina A., Monjaraz
Guzma_n E., Walusinski O. Yawning: a cue and a
signal. Heliyon. 3 (1), e00437 (2017).
- 20. Gallup, A. C. Why do we yawn? Primitive
versus derived features.
Neuroscience&BiobehavioralReviews. 35,
765-769 (2011).
- 21. Gallup, A. C., Clark, A. B. Commentary:
Yawning, acute stressors, and arousal reduction
in Nazca booby adults and nestlings. Frontiers
in Psychology. 6, 1654 (2015).
- 22. Fentress, J. C. Development of grooming
in mice with amputated forelimbs. Science. 179
(4074), 704-705. (1973).
- 23. Nicoletto, P. F. The roles of vision and
the chemical senses in predatory behavior of the
skink, Scincellalateralis. Journal of
Herpetology. 19(4), 487-491 (1985).
- 24. Fehe_r, O., Wang, H., Saar, S., Mitra,
P. P., Tchernichovski, O. De novo establishment
of wild-type song culture in the zebra finch.
Nature. 459 (7246), 564-568 (2009).
- 25. Kapita_ny, R., Nielsen, M. Are yawns
really contagious? A critique and quantification
of yawn contagion. Adaptive Human Behavior
Physiology.3 (2), 134-155 (2017).
- 26. Langford, D. J. et al. Social modulation
of pain as evidence for empathy in mice.
Science. 312 (5782), 1967-1970 (2006).
- 27. Gallup, A. C., Swartwood, L., Militello,
J., Sackett, S. Experimental evidence of
contagious yawning in budgerigars (Melopsittacus
undulatus). Animal Cognition. 18, 1051-1058
(2015).
- 28. Campbell, M. W., de Waal, F. B.
Methodological Problems in the Study of
Contagious Yawning. In The mystery of yawning in
physiology and disease. Walusinski, O. Karger
Publishers. Basel. 120-127 (2010).
- 29. R Development Core Team. R: A language
and environment for statistical computing. R
Foundation for Statistical Computing, Vienna,
Austria. ISBN 3-900051-07-0,
<http://www.R-project.org> (2009).
- 30. Moyaho, A., Beristain-Castillo, E.
Experimental Design: Basic Concepts. In
Encyclopedia of Animal Behavior., Choe, J. C.
Academic Press. Second Edition. 3, 471-479
(2019).
- 31. Urba_-Holmgren, R. et al. Genotypic
dependency of spontaneous yawning frequency in
the rat. Behavioral Brain Research. 40 (1),
29-35 (1990).
- 32. Smith, M. L., Hostetler, C. M,
Heinricher, M. M., Ryabinin, A. E. Social
transfer of pain in mice. Science Advances. 2,
e1600855 (2016).
- 33. Joly-Mascheroni, R. M., Senju, A.,
Shepherd, A. J. Dogs catch human yawns. Biology
Letters. 4 (5), 446-448 (2008).
- 34. Barthalmus, G. T., Zielinski, W. J.
Xenopus skin mucus induces oral dyskinesias that
promote escape from snakes. Pharmacology
Biochemistry and Behavior. 30 (4), 957-959
(1988).
-
-
-
-
|



