- This
amphibian shoots its mouth forwards in a
fish-like manner to suck in its
prey
-
- Pipid tadpoles of the African genus
Hymenochirus are not only among the smallest
free-swimming, feeding vertebrates, but are also
predatory suction feeders unlike other tadpoles,
which typically ingest a suspension of organic
particles. Here we use a high-speed video system
to study details of the feeding mechanics of
Hymenochirus boettgeri tadpoles and find that
they first track individual prey organisms
visually, and chase and then capture them by
mouth using suction. This feeding mechanism is
unique among frogs and is strikingly convergent
with that used by teleost fishes.
-
- Frogs of the genus Hymenochirus are unique
in that both adults and tadpoles are predatory
suction feeders. In most species of frog, adults
use their jaws, tongue or forelimbs to capture
prey, and tadpoles are usually
suspension-feeding detritivores that use
scraping mouthparts to create the suspension.
This suspension is pumped into the mouth by
rhythmic movements of the hyobranchial apparatus
(throat skeleton) , particles are trapped in the
branchial basket, and water exits through the
gill slits or a single spiracle.
-
- Hymenochirus tadpoles, however, are
morphologically divergent, lack a filter
apparatus and scraping mouthparts, and have
huge, frontally orientated eyes1. Also, they are
obligate air breathers and do not pulse-pump to
irrigate the gills like most other tadpoles.
Unaided visual observation indicates that they
are predatory carnivores, but their minute size
(the body length is less than 1 mm at first
feeding) and rapid movements make the details of
their feeding mechanics difficult to
determine.
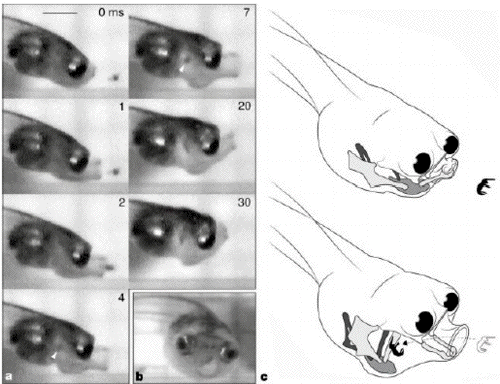 - Figure 1 Morphology and
function of suction feeding in the tadpole
Hymenochirus boettgeri(body length, 2.6 mm). a,
High-speed
- video sequence of feeding,
showing hyobranchial depression, mouth extension
and cranial elevation. Prey is engulfed within 4
ms of
- the commencement of mouth
opening. White arrowhead in frame 4 indicates
location of prey (the brine shrimp at the
nauplius stage)
- in the pharynx. Scale bar, 1
mm. b, 'Yawning'
tadpole, showing the
hydrodynamically favourable round mouth aperture
and the large,
- frontally orientated eyes.
c, Dynamics of the tadpole's feeding apparatus,
showing the mouth extension and hyobranchial
movements
- that generate suction to
ingest prey. Meckel's cartilage (lower jaw) and
the ceratohyals are shown in light grey, the
copula (basibranchial)
- is shown in medium grey, and
the ceratobranchials are shown in dark
grey.
-
- We used a high-speed video system (1,000 Hz)
with a powerful macro lens that enabled us to
follow the feeding behaviour and mechanics of H.
boettgeri tadpoles (2Ð3 mm body length;
Gosner stage 26). Our recordings reveal that
they target each prey item visually, pursue it,
then capture it by extension of a tubular mouth
during an explosive buccal expansion (Fig. 1a,
b) for movie, see supplementary information).
Tadpoles complete their mouth extension within 2
ms and engulf the prey within 4 ms; prey travel
into the mouth at 0.6 m s-1. Buccal expansion is
completed within 7 ms.Comparably sized larval
teleosts are slower feeders than these tadpoles,
taking 4Ð12 ms to engulf their prey, which
enter the mouth at 0.03Ð0.3 m s11. We
calculated a Reynolds number of 300 for prey
capture in tadpoles (from prey velocity and
tadpole mouth diameter) compared with 5Ð70
for larval teleosts. The higher Reynolds number
indicates that, although Hymenochirus is about
the same size as larval teleosts, it is faster
and better at overcoming the viscous drag that
typically confronts small aquatic
organisms.
-
- We compared our video images of moving
Hymenochirus with preserved specimens and found
that the tadpole's suction action is generated
by a combination of hyobranchial movements
(ceratohyal depression, basibranchial
retraction) and cranial elevation; downward
rotation of the lower jaw unfurls the soft
tissues that comprise the extensible mouth (Fig.
1c). After prey capture, the tadpoles expel
water slowly (over 200 ms) through the paired
gill slits of the reduced and simplified
branchial basket1 by raising the ceratohyals and
basibranchial and lowering the head to their
resting positions. The Reynolds number drops to
50 during water expulsion (Fig. 1a), and is
lower for smaller Hymenochirus tadpoles when
they begin feeding. Viscosity may thus be more
problematic during water expulsion, as has been
proposed for larval teleosts.
-
- The feeding mechanism of Hymenochirus is
remarkably like that of teleosts, which also
suction-feed by using a combination of rapid
mouth protrusion, hyobranchial depression and
cranial elevation, followed by slower water
expulsion through the gill slits. Hymenochirus
and teleosts also share a hydrodynamically
advantageous round mouth opening.
-
- Rapid mouth protrusion confers several
benefits Ñ it decreases the distance to
the prey, accelerates flow through the mouth,
restricts flow to the area in front of the
mouth, and reduces the momentum (mass *
velocity) that must be imparted to the water.
Suction feeders that have nonprotrusible mouths,
such as some firstfeeding larval fish, must suck
in more water than animals that have protrusible
mouths. They therefore generate more momentum
and suck themselves forwards. Hymenochirus
imparts little momentum to the water, resulting
in only a slight forward movement of the
tadpole's body (Fig. 1a). Miniaturized
Hymenochirus tadpoles begin feeding at a smaller
size than larval teleosts, which are universally
suction
|


