- ABSTRACT
- Yawning is a clinical sign of the activity
of various supra- and infratentorial brain
regions including the putative brainstem motor
pattern, hypothalamic paraventricular nucleus,
probably the insula and limbic structures that
are interconnected via a fiber network. This
interaction can be seen in analogy to other
cerebral functions arising from a network or
zone such as language. Within this network,
yawning fulfills its function in a stereotype,
reflex-like manner; a phylogenetically old
function, preserved across species barriers,
with the purpose of arousal, communication, and
maybe other functions including respiration.
Abnormal yawning with !3 yawns/15min without
obvious cause arises from lesions of brain areas
involved in the yawning zone, its trajectories
causing a disconnection syndrome, or from
alteration of network activity by physical or
metabolic etiologies including medication.
-
1. Introduction: what is a yawn
- Physiological yawning, i.e. yawning without
a cause in healthy individuals, is a ubiquitous
phenomenon that can be observed across species
barriers at least in most mammals (Fig. 1A, B)
if not in most classes of vertebrates (for
detailed review of published observations see
e.g. Baenninger, 1997). Yawns consist of a
typical sequence of respiratory phases such as
long inspiration, brief peak or acme, and rapid
expiration, accompanied by a coordinated motor
pattern including opening of the jaw, closure of
the eyes, contraction of facial muscles and
sometimes stretching of trunk, neck and arms.
Changes in autonomic function frequently
accompany yawning (in more detail e.g.
Baenninger, 1997). Yawning can be modulated in
its frequency by operant condition- ing, better
so in animals such as monkeys (Louboungou and
Anderson, 1987; Anderson and Wunderlich, 1988)
but also in humans (Baenninger, 1997). Its
visible action pattern - that is the coordinated
repiratory and motor behavior - has been
described as stereotyped or reflex-like
(Lehmann, 1979; Provine, 1986), because once
elicited it cannot be completely suppressed.
However, its appearance can be modulated as
well, e.g. by inhibiting opening of the jaw
(Fig. 1C) and suppressing facial and thoracic
innervation (Goessler et al., 2005). Yawning is
termed pathological, abnormal, or excessive if
it is spontaneous, more frequent than generally
perceived as normal, compulsive, and not
triggered by appropriate stimuli including
fatigue or boredom. Chasm (Latin for gap,
cavity, cleft as reviewed in lay and medical
dictionaries by Heusner, 1946) as a synonym is
per se descriptive, but rather used in abnormal
than physiological conditions. No consensus
definition exists concerning the frequency of
yawns. Descriptions vary between 2 and 30 yawns
per 10 min (Singer et al., 2007; Cattaneo et
al., 2006). We recently adopted the abnormal
yawning frequency to 3 yawns/ 15min to decrease
the likelihood that 2 subsequent, accidental
yawns were counted as one episode with abnormal
yawning (Krestel et al., 2015). Abnormal yawning
seems to be a rare neurobiological phenomenon.
Its cause in humans is unknown, but it can be
observed in a variety of medical conditions that
will be covered in more detail later on.
-
- 2. Anatomy of yawning
- 2.1. Hypotheses regarding the
neuroanatomical location of the yawning
center
- The neuroanatomical location of the motor
pattern that orchestrates yawning in humans is
not known, respectively has not been
experimentally proven. However, from early
analyses of malformed infants who lacked a
telencephalon but in whom a yawn-stretch act
could be observed, it was concluded that if a
yawn center exists, "it is to be found in the
medulla oblongata in the immediate vicinity of
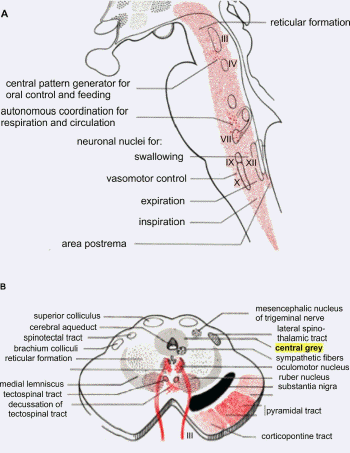
the respiratory and vasomotor centers" (Fig. 2A;
Heusner, 1946). This statement was deduced from
two earlier accounts. The first described an
anencephalic female who survived 3 months and in
whom - postmortally - the medulla spinalis &
oblongata, pons and mesencephalon were seemingly
well developed (Gamper, 1926). The telencephalon
was missing and the most cranial brain tissue
was a severely malformed diencephalon. A certain
residual function of the hypothalamus could not
be excluded because the substantia grisea
centralis behind the 3rd ventricle contained
well-formed neuronal nests however without
resemblance to known neuronal nuclei. As this
child showed phase switches between sleep and
wake, a fully developed yawn-stretch act and no
yawning nor stretching during sleep hence a
seemingly normal yawning behavior, Gamper
concluded that the yawn-stretch center is a
subcortical mechanism and should be located in
the substantia grisea centralis or
periaqueductal grey (Fig. 2B) but might well be
influenced by the telencephalon [in healthy
subjects]. The second noteworthy account was
by Catel and Krauspe (1930) who observed yawning
in a seven days surviving female anencephalus.
The nervous system was apparently well developed
up to the area around the trigeminal nerve in
the ponto-mesencephalic transition, however, the
pyramidal tract and parts of ventral pons were
missing or largely rudimentary.
-
- Cranial nerves XII to V, including their
nuclei were well formed. The rudimentary
diencephalon consisted of irregularly formed
non-coherent cellular conglomerates, cysts and
plexus-like structures. A very immature optic
tract originated from 2 of these cellular
conglomerates, which hence must have been parts
of the thalamus. However, no further anatomical
details could be delineated in this brain part.
An intact connection to the medulla oblongata
was not found. More cranial (i.e. forebrain)
venous plexus, mesenchymal cysts, partly
isolated cavities and dispersed cell nests,
reminding of rudimentary basal ganglia, were
found (Catel and Krauspe, 1930). In more recent
publications, Askenasy (1989) located the
yawning center "at the level of the reticular
brainstem close to the reticular activating
system" (RAS) and substantiated his suggestion
by case reports of patients in whom the
corticobulbar pathways were interrupted e.g. by
tumors and who were tetraplegic but could yawn.
The RAS is composed of several neuronal circuits
connecting the brainstem to the cortex. The most
caudal circuits are located in the midbrain
reticular formation and in mesencephalic and
pontine nuclei. Thus, according to today's
knowledge, Ashkenasy's yawning center would
reside somewhere in the midbrain (Fig. 2A).
Third, it was proposed that the yawning center
is located among pontomedul- lary central
pattern generators (Walusinski, 2006; Fig. 2A).
Central pattern generators are neuronal circuits
in the medulla and subserve innate, repetitive
motor behaviors that are essential for survival
such as cough, swallowing, breathing (e.g.
Marder and Rehm, 2005). Walusinski (2006)
suggested that the yawning motor can be
modulated to certain extent e.g. by (partially)
inhibited opening of the jaw (C).
 - 2.2. Hypothalamic paraventricular nucleus
controls brainstem yawning center
- In principle, the central nervous system is
composed of a "primary" and "secondary neuron".
For efferent systems this frequently means that
the "first neuron" (or network, frequently
located in the supratentorial brain) exerts some
control over the "second neuron" (or network,
frequently located in the infraten- torial
central nervous system). This central nervous
system organization is sometimes also called
top-down control. In regard to yawning, the
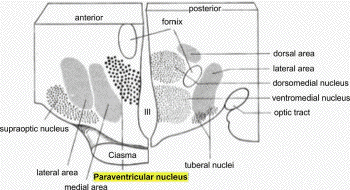
paraventricular nucleus (PVN) in the hypothala-
mus (Fig. 3) is frequently regarded as the
supratentorial control of the yawning center in
the brainstem. This leads us to a frequently
cited study in anesthetized rats, in which
stereotyped yawning could be elicited by
chemical or electrical stimulation of the PVN in
the hypothalamus (Sato-Suzuki et al., 1998). The
study proposed that parvocellular oxytocinergic
neurons in the PVN projecting to the lower
brainstem mediate the yawning response and that
nitric oxide is an important player. This
concept had already been suggested elsewhere
(see e.g. Argiolas and Melis, 1998, in 3.1.
Basic model). As the PVN might be involved in
yawning not only in rodents but also in humans,
based on conservation of the act of yawning
across species barriers at least in mammals, we
would like to review its neuroanatomy including
its connections to other brain areas and the
brainstem nuclei. In humans, the anterior part
of the hypothalamus contains neuronal nuclei of
which the most important are the preoptical,
supraoptical and the paraventricular nuclei
(Fig. 3). The PVN contains neurosecretory
magnocellular and parvocellular neurons as well
as centrally projecting (i.e. to other brain
regions) neurons. Magnocellular neurosecretory
neurons are known for their production and
transport of oxytocin and antidiuretic hormone
via their axons (supraopticohypophyseal tract)
to the posterior pituitary gland where these
peptide- hormones are secreted and exert their
neuro-endocrinological function such as oxytocin
does in regulating menorrhea, con- tractions of
the pregnant uterus, milk secretion from female
mammae and trust behavior (Kosfeld et al.,
2005).
-
- Via releasing and possibly inhibitory
hormones, parvocellular neurosecretory neu- rons
indirectly regulate the production of hormones
in the anterior pituitary gland such as growth
hormone, gonadotropins, TSH, and ACTH etc.
Finally, the PVN contains interneurons and
populations of neurons including parvocellular,
oxytocinergic neurons that project to other
brain regions. One of these projections probably
occurs via the medial forebrain bundle to
structures in the brainstem implicated in
regulation of respiratory, cardiovascular and
autonomic functions including the locus
coeruleus, solitary nucleus, ventrolateral
medulla, the motor nucleus of the vagal nerve
and via synapsing to further motor nuclei such
as the ones of the trigeminal, facial and
hypoglossal nerve (Duus, 1995). Especially the
ventrolateral medulla contains a cluster of
interneurons - named Pre-Bötzinger complex
- that seems to be essential for the generation
of respiratory rhythms in mammals (Smith et al.,
1991). The exact mechanism of rhythm generation
and transmission to motor nuclei such as the
phrenic nerve (C1-C4) innervating the diaphragm
remains controversial (Rybak et al., 2007;
Abdala et al., 2009). Further neuronal effector
centers involved in yawning are the motor nuclei
of cranial nerves V, VII, IX, XI [and XII,
more in animals?], and nerves innervating
accessory respiratory muscles (Goessler et al.,
2005). Additional data, generated in animals,
about the intranuclear organization of the PVN
and its projections is presented here, as it
might be of relevance to better understand the
neuroanatomical basis of yawning. Cells in the
parvo- and magnocellular division of the PVN
have axons that branch sometimes - so called
axon collaterals.
-
- Several axon collaterals of parvocellular
neurons ramify locally and appear to contact
dendrites of cells in both the parvo- and
magnocellular division. This evidence suggests
one possibility how the output of parvo- and
magnocellular neurons might be integrated
(Swanson and Sawchenko, 1983, and references
therein) and could be important in regard to the
hypothesis that cells in the parvocellular
division [only] mediate the controlling
effects on yawning (see 3.3. oxytocin network).
In addition, magnocellular neurons seem to
communicate by gap junctions. The predominant
projection to the brainstem is the so called
paraventriculo-spinal pathway, discovered in
1975, that was originally described to directly
connect the PVN and other hypothalamic areas
with preganglionic cell groups of both the
parasympathetic and sympathetic divisions of the
autonomic nervous system in the dorsal vagal
complex and thoracic spinal cord, respectively.
Subsequent work by several groups added more
detail to its course. In cow and rat, fibers
originating in the PVN descend initially through
the median forebrain bundle and then, after
passing between substantia nigra and red
nucleus, continue through ventrolateral parts of
the reticular formation to enter the
dorsolateral funiculus of the spinal cord. In
the pons, fibers leave this pathway to innervate
the parabrachial nucleus and the locus
coeruleus, while in the medulla, some fibers
arch dorsally to innervate the dorsal motor
nucleus of the vagal nerve and the nucleus
tractus solitarius. In addition, a second
pathway descends through the central gray and
appears to innervate the Edinger Westphal
nucleus, locus coeruleus, and perhaps the
central gray (substantia grisea centralis), as
well (Swanson and Sawchenko, 1983 and references
therein). This detailed description also
contains reported fibers to neuronal networks
suggested to home the putative yawning motor
pattern including the central or peri-
aqueductal grey (Gamper, 1926), the midbrain
reticular formation near the reticular
activating system (Askenasy, 1989), and the
ponto-medullary brainstem near the reticular
formation (Walusinski, 2006).
-
- 2.3. Trajectories projecting to the
PVN
- Most of neural afferents to the PVN origin
from a rather small number of cell groups in the
brainstem, hypothalamus, and limbic regions of
the telencephalon. The brainstem sends
noradrenergic and adrenergic fibers to the PVN,
which form one of the most dense catecholamine
terminal fields in the brain. Mainly
noradrenergic fibers project from A2
[noradrenergic] (and C2
[adrenergic]) cell groups of the nucleus
tractus solitarius and from the A6 group of
locus coeruleus to the parvocellular division,
and from A1 (and C1) cell groups of the ventral
medulla (located dorsal to but not within the
lateral reticular nucleus) to both the parvo-
and magnocellular division (Swanson and
Sawchenko, 1983 and references therein).
-
- Sparse serotoninergic afferents reach the
PVN mainly from mesencephalic raphe nuclei. The
hypothalamus projects to the PVN from the
anterior and lateral hypothalamic area, the
ventromedial and dorsome- dial nucleus, and the
preoptic area which all end in the parvocellular
division. Only projections from the dorsomedial
nucleus and the median preoptic nucleus appear
to end in the magnocellular division, as well.
The suprachiasmatic nucleus, which receives
direct input from the retina, projects to the
parvocellular part. ACTH immunoreactive fibers
are found in the parvocellular divison and in
the magnocellular division where oxytocinergic
cells are concentrated. These projections arise
from immunoreactive neurons in and near the
arcuate nucleus of the hypothalamus. Because
b-endorphin has been co- localized within many
ACTH-stained neurons, b-endorphin- containing
fibers also reach the PVN (Swanson and
Sawchenko, 1983 and references therein). The
telencephalon may influence PVN activity, but
rather through its limbic system.
-
- There is as yet no evidence for direct
projections from neocortical areas to the PVN.
Retrograde transport studies in the rat suggest
that the lateral nucleus of the septal region,
the amygdala (medial nucleus), and the
hippocampus (specifically the ventral part of
the subiculum) project to the PVN. However,
these results do not seem to be without
controversy (see Swanson and Sawchenko, 1983 and
references therein). Other sources support
[oxy- tocinergic] connections between
the hippocampus, tuberomam- millary bodies, and
the PVN (e.g. Walusinski, 2006; Argiolas and
Gessa, 1991). The bed nucleus of the stria
terminalis may present one additional route by
which the limbic region may influence
parvocellular and oxytocinergic magnocellular
neurons in the PVN (Swanson and Sawchenko, 1983
and references therein). Future work should be
directed into validating the biochemical nature
of fibers to the putative yawning motor pattern,
given the fact that circa 30 different putative
neurotransmitters have been identified in cell
bodies or in presumed terminals within the PVN
(Swanson and Sawchenko, 1983).
-
- 3. Neurochemistry of yawning
- 3.1. Basic model with yawning as
oxytocinergic behavior
- A lot of work has been invested into
elucidating the neurochemistry involved in
yawning. The subsequently presented overview
centers on a model, proposed by a group that
contributed significantly to this field and also
integrated work of many others (Argiolas and
Melis, 1998). Their model is extended and
updated with unmentioned and more recent
findings. The basic model suggests that
oxytocinergic neurons originating in the PVN of
the hypothalamus and projecting to
extra-hypothalamic brain areas mediate the
expression of yawning in animals in many but not
all circumstances. Activation of neurons in the
PVN by dopamine receptor agonists, excitatory
amino acids, and oxytocin results in yawning,
while their inhibition by e.g. opioids prevents
the behavioral response. Dopamine receptor
agonists bind mainly to D2 receptors and
oxytocin to uterine-type oxytocinergic receptors
and this leads to activation of
[omega-conotoxin-sensitive] N-type Ca2+
channels via [pertussis toxin-sensitive]
G0/Gq proteins and subsequently to calcium
influx. To a minor extent, Ca2+ is released from
intracellular stores via the
Phosphoinositid-Phospholipase C (PLC) pathway.
Excitatory neurotransmitters including glutamate
and N-Methyl-D-aspartic acid (NMDA), but not
a-amino-3- hydroxy-5-methyl-4-isoxazolepropionic
acid (AMPA), were origi- nally suggested to
activate NMDA receptors, which are coupled to
Ca2+ channels. This was confirmed by subsequent
agonist- antagonist studies showing that
NMDA-induced yawning was antagonized by
(+)-MK-801, a selective antagonist of NMDA
receptors, but not by omega-conotoxin, a potent
blocker of N- type Ca2+ channels (Succu et al.,
1998), and it is therefore likely that NMDA
receptors mediate Ca2+ influx themselves.
Increased cytoplasmic Ca2+ concentration in
oxytocinergic neurons activates nitric oxide
synthase (NOS) to produce nitric oxide (NO) that
is suggested to act as intracellular messenger.
The mechanism by which NO activates
oxytocinergic transmission is not known.
Guanylate cyclase, one favored target of NO,
seemed then not involved. The inhibitory effect
of opioids such as morphine on yawning induced
by dopamine D2 receptor agonists, oxytocin, or
NMDA is apparently mediated by m-type morphine
receptors since the morphine effect is blocked
by the prior administration of the respective
antagonist naloxone. In addition, a very potent
agonist acting at kappa opioid receptors is
ineffective in eliciting yawning. Signal
transmission from extra - to intracellular may
be mediated by decreased intracellular Ca2+ and
reduced NOS activity, as less NOS metabolites
were measured by in vivo microdialysis in the
PVN after administration of morphine. The link
between m-recep- tor activation and decreased
intracellular Ca2+ was stated as unknown by
Argiolas and Melis (1998), but is probably due
to closure of voltage-gated Ca2+ channels (e.g.
Schroeder et al., 1991). Finally, it was
suggested that the PVN is apparently not
involved in yawning induced by 5-HT2C agonists
or ACTH/MSH. It was further proposed that the
latter compounds act at sites located [prior
to or] after oxytocinergic neurons, but
there exist probably additional neuronal
pathways that influence yawning. This was also
proposed for cholinergic, noradrenergic &
GABA-ergic systems involved in yawning (Argiolas
and Melis, 1998).
-
- 3.2. Dopaminergic modulation of the basic
model
- The PVN receives dopaminergic afferents,
which was concluded from the presence of
dopaminergic fibers in the vicinity of parvo-
and magnocellular neurons of the PVN and from
dopaminergic boutons synapsing on magnocellular
neu- rons. The origin of these fibers was not
precisely located but the ventral tegmental area
was suggested (Buijs et al., 1984). The
- basic model by Agriolas et al. suggested
that the dopaminergic effect of yawning is
mediated by postsynaptic dopamine D2 but not D1
receptors. The postsynaptic site of dopamine
receptor activation was later confirmed (Collins
and Eguibar, 2010). Recently, the dopamine D2
receptor was questioned and the dopamine D3
receptor was suggested instead. This was based
on agonist-antagonist studies in rats (Collins
and Woods, 2008; Collins et al., 2008; Baladi et
al., 2010). The same group went on to propose
specific roles for the D3 and D2 receptor in
dopamine agonist-induced yawning (Collins and
Eguibar, 2010). Other experimental data (again
in rats) did not support a major role of
dopamine D3 and D4 receptors, but of D2
receptors in dopamine agonist-induced yawning
(Depoortère et al., 2009; Sanna et al.,
2012a). There is evidence that the D2 receptor
still might play a role in dopamine
agonist-induced yawning. This comes from
treatment of humans with Parkinson's disease
(PD). In these patients, apomorphine, a
relatively non-selective dopamine receptor
agonist, with possibly slightly higher affinity
for D2-like dopamine receptors, is used to test
dopaminergic responsiveness or intermittently
treat parkinsonian motor fluctuations. Yawning
was quite commonly observed after apomorphine
administration (Frankel et al., 1990; O'Sullivan
et al., 1999 and references therein). Four major
dopaminergic pathways have been described in
mammalian brain. These are the mesocortical and
limbic pathways with their dopamine- producing
somata in the ventral tegmental area, the
nigros- triatal pathway and the
tuberoinfundibular pathway with its
dopamine-containing somata in the hypothalamus.
To what extent the nigrostriatal system is
involved in yawning, remains to be answered.
However, microinjection of nanogram amounts of
apomorphine and other dopamine D2 agonists into
the PVN but not in the striatum of rats induced
yawning (Melis et al., 1987). Apart from
possible involvement of several receptor
subtypes in yawning, dopamine receptors seem to
underlie diurnal variations in sensitivity,
since yawning, induced with identical doses of
apomorphine, could be elicited more frequently
in the morning than in the evening in healthy
men (Lal et al., 2000). Dopamine-agonist induced
yawning can be modulated by other transmitters.
These include nicotine (Tizabi et al., 1999;
Brown et al., 2006), adenosine (Rimondini et
al., 1998), endocannabinoids (Beltramo et al.,
2000; Nakamura-Palacios et al., 2002), and
noradrenaline (Sawchenko and Swanson, 1981;
Nowak et al., 2006). A dense catechol- aminergic
innervation was shown to synapse on PVN neurons
(e.g. Swanson et al., 1981). Of interest is also
the modulatory effect of dexamethasone, since
yawning has been postulated - amongst others- as
a signal for stress (see Communication
hypothesis, below). Dexamethasone modulated
(increased as single and decreased as repeated
administration) pilocarpine- but not
apomorphine-induced yawning in rats
(Hipólide et al., 1999).
-
- 3.3. Oxytocin network extending to supra-
and infratentorial brain
- Oxytocin immunoreactivity is found in
magnocellular (e.g. Buijs, 1978; Hatakeyama et
al., 1996) and in parvocellular neurons (e.g.
Buijs et al., 1978). The presence of NOS,
indicated by positive NADPH diaphorase staining,
colocalizes with oxytocinergic mag- nocellular
neurons (e.g. Sánchez et al., 1994) and
is also found in the rostral part of the PVN
where rather parvocellular neurons reside (e.g.
Sato-Suzuki et al., 1998). However, a direct co-
localization of NOS and oxytocin in
parvocellular neurons escaped our literature
review. The type of oxytocineric PVN neuron
involved in yawning has not been unequivocally
proven, but reports of recent years favor
parvocellular neurons (see e.g. Sato- Suzuki et
al., 1998; Kita et al., 2006).
Neuroanatomically, both parvo- and magnocellular
neurons were shown to have extra- hypothalamic
projections. Oxytocin-containing fibers from
mag- nocellular PVN neurons were traced to the
dorsal/ventral hippocampus, amydgala, substantia
nigra, central gray, nucleus tractus solitarius,
and nucleus ambiguus in rats. In contrast to
projections to rostral brain areas, far more
oxytocin- than vasopressin-containing fibers
were found in the medulla oblongata (and spinal
cord) (Buijs, 1978). The author even suggested
that some of the bi- and multipolar PVN cells
may project to the neurohypophysis, as well as
to extrahypothalamic areas and added that it is
not precisely known whether all of these
extrahypotha- lamic fibers are axons or whether
some might be "dendrites with a receptive
function" (Buijs, 1978). In contrast, retrograde
double- labeling studies from another group
indicated that hypothalamic projections to the
brainstem or spinal cord do not, for the most
part, arise as collaterals from magnocellular
PVN cells that also project to the posterior
pituitary gland (reviewed in Sawchenko and
Swanson, 1982). Their retrograde labeling
studies found roughly 10 times more cells in the
parvo- than magnocelluar disvision of the PVN
that contained a retrograde tracer (injected
into the dorsal motor nucleus of the vagus and
nucleuse of the solitary tract) and were
immunoreactive for oxytocin (Sawchenko and
Swanson, 1982). In a follow-up study - by
injecting a retrograde tracer into the dorsal
vagal complex and thoracic spinal cord and
staining the PVN immunocytochemically against
oxyto- cin and vasopressin - fibers within the
paraventriculo-spinal pathway could be
identified that originated predominantly from
oxytocinergic cells, located in the parvo- and
magnocellular division of the PVN but more
frequently in the medial and lateral
parvocellular division (Swanson and Sawchenko,
1983).
-
- However, only 20% of retrogradely labeled
cells stained positive for either oxytocin or
vasopressin suggesting that additional cell
types contributed to this tract. Interestingly,
additional 5% of cells containing the retrograde
tracer stained immunocytochemically positive for
tyrosine hydroxylase (suggested by the authors
to be dopaminergic), somatostatin, and
met-enkephalin. Apart from this tract connecting
the PVN with preganglionic cells of parasympa-
thetic and sympathetic divisions of the
autonomic nervous system, no statement was made
about the nature of fibers that branch from the
paraventriculo-spinal pathway to putative
neuronal networks containing the yawning motor
pattern. Still, fibers from magno- cellular PVN
cells also project to the vicinity of putative
cardiovascular and respiratory nuclei. Several
experimental studies in rats showed that
electrical or chemical stimulation of the
parvocellular division of the PVN affected
yawning (e.g. Sato- Suzuki et al., 1998; Kita et
al., 2000). However, the PVN is so small in
these animals and even if targeting was precise,
it cannot be excluded that magnocellular neurons
in the vicinity are excited as well. Therefore,
the issue might not be finally settled which
type of PVN neuron is involved in yawning
control. The hippocampus was repetitively
implicated in yawning as oxytocin injections
into it induced yawning (e.g. Argiolas and
Gessa, 1991). These observa- tions might reflect
an indirect effect using the PVN as sort of
relay station, as hippocampal-hypothalamic
oxytocinergic projections were shown to exist.
Similarily, oxytocin may induce yawning if
injected not only in the PVN and hippocampus,
but also in other brain areas, such as the
ventral tegmental area and the posterior nucleus
of the amygdala that, like the hippocampus,
receive oxytocinergic projections from the PVN
(Sanna et al., 2012b). Oxytocin seems to act as
neurotransmitter in the brainstem as
iontophoretically applied oxytocin is able to
change the firing rate of some neurons in the
dorsal medulla and oxytocin is released in Ca2+
dependent manner from tissue slices of medulla
upon potassium application (Swanson and
Sawchenko, 1983). The putative link between the
action of NO as intracellular messenger and
oxytocinergic transmission in yawning is still
missing.
-
- 3.4. N-Methyl-D-aspartic acid (NMDA) and
gamma-Aminobutyric acid (GABA)
- A correlation between NMDA-induced yawning
and NOS in the PVN was suggested. First,
NMDA-induced yawning in freely moving conscious
rats could be blocked by a NOS inhibitor (Melis
et al., 1994a). Second, rats that had been
rendered diabetic and impaired in their
NMDA-induced yawning response by strepto-
zotocin treatment were injected adenoviruses
carrying a gene for neuronal NOS into the PVN.
This could restore NMDA-induced yawning (Zheng
et al., 2007). Excitatory (glutamatergic) amino
acid activation is rather mediated via NMDA-
thanAMPA receptors, as administration of NMDA
but not AMPA into the PVN induced yawning (Melis
et al., 1994b; Collins and Eguibar, 2010).
Yawning induced by injection of a NO-releasing
compound or glutamate into the medial
parvocellular PVN of anesthetized rats was
paralleled in its effect by injection of
cyanide, a mitochondrial cytochrome c oxidase
inhibitor. This led to the suggestion that the
medial parvocellular division is sensitive to
chemical hypoxia or ischemia and even contains
an oxygen sensor that causes yawning (Kita et
al., 2000; see also 4.1. Respiratory
hypothesis). GABA may also modulate yawning by
affecting NOS activity. Injection of GABA-A
(muscimol) but not GABA-B (baclofen) receptor
agonists into the PVN reduced yawning induced by
apomorphine, NMDA, and oxytocin and was
paralleled by a decrease in NOS metabolites as
measured in a paraventricular microdialysate
(Melis and Argiolas, 2002). However, this effect
is controversal (see Doger et al., 1989).
-
- 3.5. Endogenous opioids
- Enkephalins, being endogenous opioids, are
found in magno- cellular and parvocellular PVN
divisions, and the paraventriculo- hypophyseal
tract may also contain enkephalins (Swanson and
Sawchenko, 1983). Yawning is a prevalent sign of
the opiate withdrawal syndrome in human opiate
addicts (O'Brien, 1996; reviewed in Argiolas and
Melis, 1998). Adenosine and opiate systems were
shown to modulate each other, resulting in a
withdrawal syndrome including yawning, if rats -
physically made dependent with adenosine
agonists - were challenged with adenosine
antagonists, or if adenosine antagonists were
given to morphine pre-treated rats (Coupar and
Tran, 2001). Intracellular phosphorylation,
converting the opioid receptor into a constitu-
tively active form, plays a role in the opiate
withdrawal syndrome in dogs. Administration of a
protein kinase C inhibitor, prior to rendering
dogs opioid dependent, attenuated acute
symptoms, if withdrawal was induced (Freye and
Levy, 2005).
-
- 3.6. Serotonergic yawning
- Serotonin (5-HT) 2C receptor agonists are
effective at inducing yawning in rats and
humans, which can be blocked by 5-HT2C
antagonists. Stimulation of 5-HT1A receptors can
also block dopamine induced yawning (Argiolas
and Melis, 1998). The relationship between
serotonin and yawning is however not
straightforward in rats: 5-HT2C receptor
agonists do not induce yawning when they are
directly injected into the PVN; 5-HT2C receptor
antagonists do not prevent dopamine/oxytocin
induced yawning and 5-HT2C mediated yawning is
not prevented by oxytocin receptor antagonists
but by NOS inhibitors, when given into lateral
ventricles (Argiolas and Melis, 1998). In
addition, 5-HT6 receptors play a role in the
control of yawning, as respective antagonists
could increase the number of yawns (Sleight et
al., 1998), but not in continuous treatment for
seven days (Marcos et al., 2008). In humans,
yawning was reported as side effect during
treatment with selective serotonin reuptake
inhibitors such as paroxetine (Harada, 2006),
escitalopram (dosage reduction termi- nated
yawning) (Gutiérrez-Alvarez, 2007), the
combined serotonin and norepinephrine reuptake
inhibitor Duloxetine (De Las Cuevas and Sanz,
2007) and with venlafaxine, a reuptake-
inhibitor of serotonin, norepinephrine and
weakly dopamine (Chen and Lu, 2009). Its site of
action was proposed to lay outside the PVN and
we recently proposed that the insula might be
the long sought-after brain region for
serotonin-mediated yawning (see also 5.1.1.
Anterior circulation stroke; Krestel et al.,
2015).
-
- 3.7. Acetylcholine and
noradrenaline
- Cholinergic or mimetic drugs such as the
acetylcholine esterase inhibitor physiostigmine
and the muscarinic receptor agonist pilocarpine
can induce yawning in rodents. This effect seems
to be mediated by M1- and M2 subtypes of
muscarinic acetylcholine receptors (AChR) as the
muscarinic receptor antagonists atropine and
scopolamine, but not nicotinic AChR antagonists
prevented yawning induced by ACTH, dopaminergic
agonists, and oxytocin (Argiolas and Melis,
1998). It is not known at which neuroanatomical
site acetylcholine and its agonists affect
yawning. Possibilities include modulation of the
PVN afferents, efferents and at somata of PVN
neurons themselves. Lesion studies suggested a
central role of septo-hippocampal cholinergic
neurons in the induction of cholinergic yawning,
but it remained unclear which trajectories
reached the brainstem to induce the yawning
motor pattern (Collins and Eguibar, 2010). The
above mentioned observation that muscarinic
receptor antagonists prevented yawning induced
by above mentioned transmitters and hormones
suggests that modulation takes place at the
level of PVN efferents or directly in the
brainstem. Indeed, muscarinic AChR subtypes
M1-M5 have been found in pons and medulla of
rats with biochemical methods (Wei et al.,
1994). Even the nucleus tractus solitarius
(Endoh, 2007; patch clamp experiments) and the
rostral ventrolateral medulla (Kumar et al.,
2009; quantitative RT-PCR) were shown to express
M2 type muscarinic AChR in rat. The proof
however is still missing whether cholinergic
trajectories from the PVN to brainstem exist
that may directly induce yawning. On the other
hand, as intense bidirectional noradrenergic
projections between the parvocellular division
and the brainstem (locus coeruleus, nucleus
tractus solitaries, dosal vagal complex) exist
(e.g. Sawchenko and Swanson, 1981), adrenergic
modulation of yawning via direct PVN brainstem
projections seems possible.
-
- 3.8. Adrenocorticotropic hormone (ACTH)
and melanocyte- stimulating hormones
- Adrenocorticotropic hormone (ACTH) and
melanocyte-stimu- lating hormones (MSH) can
induce a stretching-yawning syn- drome that was
considered different from the classic yawning
syndrome (induced e.g. by dopamine agonists or
oxytocin), because the behavior starts only 20
to 30 min after intraventricular injection and
lasts for hours. Some evidence concerning the
neuroanatomical site of action comes from a
study in which injection of ACTH into the peri-
[not para-] ventricular area around the
3rd ventricle could induce yawning and be
blocked by prior installation of a melanocortin
4 receptor antagonist. However, yawning could
not be elicited by injection into CA1 area of
hippocampus, caudate nucleus or preoptic area
(Argiolas et al., 2000). The involvement of PVN
- brainstem projections in ACTH/ MSH induced
yawning remains controversial. It shows in some
instances analogies to dopamine or
oxytocin-induced yawning such as prevention of
yawning by omega-conotoxin and NOS inhibitors
and hypophysectomy. On the other hand ACTH/MSH
induced yawning is not prevented by PVN lesions
nor by dopamine or oxytocin receptor antagonists
(reviewed in Argiolas and Gessa, 1991).
Therefore, both possibilities- i.e. involvement
and detouring the PVN in yawning - seem
possible.
-
- 4. Potential roles of yawning
- 4.1. Respiratory hypothesis
- Besides unraveling the neuroanatomical and
chemical blue- print of yawning, it has always
been of interest to understand its role in
biology and why it has been evolutionary
conserved. One of the earlier and still widely
held beliefs is that yawning is due to changes
in respiratory function or brain perfusion (with
oxygen), respectively. The respiratory
hypothesis dates back to Johannes de Gorter
(1689-1762), a prolific Dutch author who, in his
book "De Perspiratione insensibili", attributed
yawning "to a need for faster blood circulation
and to cerebral anemia". More recently, H.
Russell's monograph from 1891, which is one of
the earliest and most extensive printed excerpts
about yawning in the English language, suggests
such physiological power for yawning. Herein,
yawning is an automatic impulse caused by bad
air in the lungs, designed by nature as a
gymnastic and intended both to awaken
respiratory organs into activity and to effect a
stimulation of the brain through increased
activity of the circulation (Russell, 1891).
Although this concept has been frequently
attacked and seems to date the least
experimentally validated hypothesis about the
purpose of yawning, it is still not without
favor among clinicians. Often cited together is
the theory that yawning is caused by anemia or
insufficient perfusion of the brain. One of its
early protagonists was Valentin Dumpert who
suggested that yawning is not only caused by
anemia or poor brain circulation, but in
addition a tendency to defend impaired
consciousness is required [For him the yawn-
stretch act was an elementary indirect vascular
reflex ("elemen- tarer indirekter
Gefässreflex, dem das ganze
Blutgefässsystem unterstellt ist")]
that controlled all blood vessels (Dumpert,
1921). Hence, the two theories together can be
formulated these days as the hypothesis of an
impact of (cerebral) hypoxemia or hypercap- nia
on yawning frequency and duration.
-
- Observations in favor of this hypothesis
have been multiple such as: i) Enhancement of
venous backflow to the heart and activation of
the parasympa- thetic nervous system with
dilation of arterioles and bronchioli during
yawns results in increased respiratory
elimination of carbon dioxide and uptake of
oxygen (reviewed in Askenasy, 1989). ii) Deep
inspiration removed atelectasis that accumulated
during 2 h of normal ventilation in excised rat
lungs by a single large inflation and was
assumed to parallel the in vivo situation with
shallow breathing and subsequent yawning (Thet
et al., 1979). iii) Brain hypoperfusion by
stepwise reduction of arterial blood pressure
triggered yawning and decrease in pO2 and EEG
frequency (Karasawa et al., 1982). iv)
Experimentally induced chemical hypoxia by local
injection of cyanide, a mitochondrial cytochrome
c oxidase inhibitor, into the parvocellular part
of the PVN elicited yawning in anesthetized
rats, prompting the authors to suggest that this
area contains an oxygen sensor (Kita et al.,
2000). Indeed, current understanding of
candidates in the carotid body - supposed to
register oxygen fluctuations in blood - include
NADPH oxidases generating reactive oxygen
species in O2 dependent manner, oxygen-regulated
plasmalemmal K+ channels, and cytochrome c
oxidases which can respond with depolarization
of mitochondrial membranes and Ca2+ release
(Kummer and Yamamoto, 2002). However, the
currently favored model for oxygen sensing in
the carotid body is inhibition of O2 sensitive
K+ channels in glomus cells with subsequent
depolarisation, Ca2+ entry, and transmitter
release activating afferent nerve fibers
(López-Barneo et al., 2008). Evidence
against the respiratory hypothesis include
observations of yawns - mouth movements,
grimaces, tongue protrusions - as early as in
human and rodent fetuses (Van Woerden et al.,
1988; Sherer et al., 1991; Walusinski,
2010).
-
- Although fetuses require oxygen and must
expel carbon dioxide, they clearly do not use
the pulmonary respiration mechanism. Fetal mouth
movements, grimacing and stretching might be the
exercise of a motor pattern by which neonates
are able to produce normally integrated yawns
within minutes after being born. Second, a study
in young, healthy students in whom neither
yawning rate and/or duration was altered by
breathing gas mixtures with higher than normal
levels of CO2 (3 or 5%) or 100% O2, although
both affected breathing rate. Physical exercise
sufficient to double breathing rate had no
effect on yawning, too. The authors concluded
that altered partial pressures of O2 and CO2 in
blood did not affect yawning (Provine et al.,
1987). However, the report does not mention any
measurements of blood gas partial pressures
(neither transcutaneous nor arterial). Third,
periods of apnea are not followed by
compensatory yawning after breathing is resumed
(reviewed in Baenninger, 1997). The
measurements, recorded by one of us using
digital oxymetric monitoring, show a light drop
of oxygen saturation immediately after a yawn
(unpublished data). The conclusion seems to be
that fluctuations in partial pressure of oxygen
or carbon dioxide do not seem to be a driving
force for yawning in humans. Still, to fully
exclude the respiratory hypothesis, the study by
Provine et al. (1987) should probably be
repeated with measurements of O2 and CO2 partial
pressures.
-
- 4.2. Communication hypothesis
- The second so-called communication
hypothesis is about the purpose of yawning as a
communicative tool that serves to synchronize
the behavior of a group (Daquin et al., 2001).
One of the conclusions in an analysis of a novel
by Jean-Paul Sartre about profound boredom gets
it to the point: "the yawn is a hole with a
difference [...] it does not invite
filling [...] butitisfulland expressive"
(Bell, 1980). It is generally accepted that
yawning signalizes sleepiness. This association
is obvious and has been proven as yawning occurs
more frequently in mornings and evenings during
transitions between activity and sleep (Greco et
al., 1993; Baenninger, 1997). Furthermore, it is
presumed to signalize lack of interest/boredom,
as increased yawning rates were observed in
students sitting in a class, in people driving a
car or during studying (which is generally but
not necessarily believed to be boring; Greco et
al., 1993). In addition, more frequent yawning
is associated with viewing uninteresting,
repetitive stimuli than with viewing interesting
stimuli (Provine and Hamernik, 1986). Yawning as
a signal for stress or threat/conflict is more
established in the animal kingdom (reviewed in
Baenninger, 1997). In fact, increased yawning
frequency as a signal for stress in humans was
reported only once in a survey of students
(Greco et al., 1993).
-
- 4.2.1. Contagious yawning
- Apart from yawning without obvious external
trigger, it can be elicited by observing or
listening other individuals yawn and by reading
or thinking about it. The urge to yawn is often
irresistible and only partially suppressible and
has hence been termed contagious. Its
neuroanatomical substrate has been attempted to
be unraveled with functional magnetic resonance
imaging (fMRI) in humans. Differences in
fMRI-activated brain areas were found among
experimental studies. According to the
predominantly activated brain region - and
results of non-fMRI studies - different theories
have been proposed. First, contagious yawning
may be mediated by cerebral mechanisms involved
in empathy and self- processing because brains
of probands observing other people yawn versus
laugh were selectively activated in areas that
are believed to be involved in processing of
empathy (theory of mind) and information about
oneself such as the posterior cingulate,
precuneus, bilateral thalamus, and
parahippocampal gyrus (Platek et al., 2005). The
same group also showed that the urge to yawn in
probands observing others yawn was amongst
others positively correlated with scores of
pencil tests for empathy (Platek et al., 2003).
Support for this theory came from another group,
showing that the ventromedial prefrontal cortex
(vmPFC) is selectively activated in fMRI scans
of brains of people observing yawn versus
cough/gape conditions (Nahab et al., 2009). The
vmPFC was shown to be involved in empathic
processing (Eslinger, 1998; Shamay- Tsoory et
al., 2003), but also in weighing or biasing
future choices and minimizing decision-making
time (Bechara et al., 1999, 2000; Fellows and
Farah, 2007).
-
- A different mechanistic hypothesis is that
contagious yawning is mediated by a mechanism
called action understanding or imitation. This
theory was put forward by a study that compared
yawning and intransitive orofacial movement
conditions. Here, selective fMRI activation was
seen in the superior temporal sulcus (STS;
Schürmann et al., 2005). Neurons in the STS
discharge when an individual observes certain
movements but not when the individual performs
the corresponding motor actions him-/herself. As
one characteristic of mirror neurons is to
discharge during perception of a motor action
but - as they are motor neurons - also during
performance of that action by the individual
him-/herself and because STS neurons lack motor
neuron properties, they are considered to be
strictly related to the mirror neuron system but
not part of it (Rizzolatti and Craighero, 2004).
The STS is believed to be the area where the
action made by another individual is compared
with sensory-motor consequences of the same
action made by oneself in order to copy a new
action or to adjust an action present in one's
motor repertoire to a different action (Iacoboni
et al., 2001). Therefore, the unique STS-
activation in Schürmann's study might
reflect a [pattern] recognition process
of the yawning versus orofacial movement
conditions. Taken together, the above-mentioned
fMRI studies have in common that their yawning
study conditions were based on seeing films or
pictures of other subjects and evoked unique
activation in brain areas that do not belong to
the mirror neuron system.
-
- Therefore, the authors univocally concluded
that contagious yawning is not related to
imitation that is mediated by the mirror neuron
system. They rather suggest the cortical release
of a stereotypical motor pattern (in particular
Schürmann et al., 2005; Nahab et al.,
2009). In contrast, investigation of auditory
contagious yawning by fMRI showed that yawn
sounds not only were effective at eliciting an
urge to yawn but yawn sounds with high urge to
yawn significantly activated essential parts of
the mirror neuron system including right
posterior inferior frontal gyrus (pIFG) and
posterior superior temporal gyrus (STS; Arnott
et al., 2009). How can this controversy about
involvement of the mirror neuron system in
contagious yawning be explained? First, the
stimulus may make a difference, as processing of
sound takes place in a different cortical area
than processing visual stimuli. However, when it
comes to recognition of a stereotypical pattern,
acoustically and visually processed information
should converge. Here, the mirror neuron system
offers the theoretical advantage of containing
so called visuo- motor and echo-neurons; cells
that discharge upon seeing or hearing a
particular action (Rizzolatti and Craighero,
2004) which makes them in principle suitable to
recognize different sensory information of one
action. On the other hand, fMRI activation in
classical mirror neuron areas including the
posterior inferior frontal gyrus can be detected
in the studies by Schürmann et al. (2005)
and Nahab et al. (2009) especially on second
examination of those figures in which yawning
was compared to a blank screen or white cross on
grey screen respectively, and not to orofacial
movements or gape/cough conditions. Even the
study of Platek et al. (2005) showed figures
with fMRI activation in the vicinity of the
superior/inferior parietal gyrus - being part of
the mirror neuron network - if yawning was
compared to neutral face conditions and not to
laugh. Finally, the studies about visual
contagious yawning did not grade the urge to
yawn of their probands in response to visual
stimuli (exept for Nahab et al. whose probands
answered a binary questionnaire whether they
yawned while seeing visual stimuli and whether
they yawned more when seeing repeated
stimuli).
-
- Therefore correlations of subjective
high-urge-to-yawn feelings with fMRI activity
patterns were not reported. In summary, the
mirror neuron network can still be involved in
the recognition and processing of contagious
yawning. The question remains whether contagious
yawning is based on imitation, action
understanding, empathy, or some "inborn"
capacity of the brain. One relative argument
against imitation is an observation by Moore
(1942) that blind people yawn in response to an
audio recording of yawns. How can blind people
perform the act of yawning in a correct and
recognizable way, if they haven't ever observed
it and if it is not a motor pattern belonging to
the basic repertoire of the brain? It is
therefore suggested that the efferent part of
contagious yawning is not elicited by cortical
mirror neuron motor areas. Rather the highly
stereotypical expression is recognized by the
cortex (might it be STS, pIFG or vmPFC) and
might activate intermediate control centers
including e.g. the insula, hippocampus, and the
PVN which then induce execution of the motor
program by brainstem structures that coordinate
innervation of facial, laryngeal, pharyn- geal
structures, the diaphragm and accessory
respiratory muscles. As mentioned, contagious
yawning does not necessarily depend on visual or
acoustic input only. Thinking or reading about
yawning can be enough to trigger the yawn act
(Baenninger and Greco, 1991; Greco et al., 1993;
Provine, 1986). The mirror neuron network would
be handy to explain this sort of observation in
its function as pattern recognition system. The
theory of speech evolution is based on a
transfer of gestural meaning to abstract sound
meaning and on the existence of a common neural
substrate for hand/arm and speech gestures, as
well as for sound and language processing
(Rizzolatti and Craighero, 2004). It was shown
that during reading and spontaneous speech
without further motor action, mirror neuron
areas including the hand motor cortex were
activated as well (Meister et al., 2003).
Therefore, reading or thinking about yawning
might activate brain areas that started
evolutionary as motor neurons and have become
specialized to recognize, that is to discharge
to, the written perception of an action or its
retrieval from memory but may not necessarily
contribute to the motor performance of that
action anymore. The conclusion is that the
communication hypothesis can only explain a part
of the behavior by great apes, perhaps parrots
and other birds, and mammals, but not by
reptilians. The sauropsids include reptiles
(poikilotherm) and birds (homeotherm). But, in
fact, relative brain size varies greatly among
sauropsids, with turtles and snakes occupying
the low end of the range. All reptiles present a
trilaminar cortex in a large dorsal ventricular
ridge that protrudes into the ventricle. It is
difficult to homologize to anything in mammalian
brains. These differences in cerebral
architecture probably explain why social
processes such as unconscious mimicry like
contagious yawning cannot be observed by
reptiles or tortoises (Wilkinson et al.,
2011).
-
- 4.3. Arousal hypothesis
- The third major hypothesis favors arousal as
the prominent biological purpose of yawning.
Supporters of this hypothesis include Ronald
Baenninger who recapitulated that "the thread
used to tie all the diverse data and
observations together is the basic hypothesis
that a major function of yawning is to regulate
levels of arousal" (Baenninger, 1997). For his
conclusion, he also reverted to own data such as
the study showing significantly increased wrist
activity as measured with wrist monitors after
yawning as an indicator for elevated arousal
(Baenninger et al., 1996). While analyzing the
biochemistry of yawning, the constellation of
neurotransmitters favoring yawning such as
serotonin and dopamine suggested to JAskenasy
(1989), another protagonist of the arousal
hypothesis, that yawning has an "antisleep
effect". Olivier Walusinski suggested that the
yawning-stretch syndrome may be an afferent
proprioceptive feedback contributing to one's
cerebral body scheme. But he also advocated its
contribution to arousal by triggering
dissolution of the cerebral network control-
ling sleep - and especially REM sleep - and
facilitating the establishment of a network that
controls the wake status (Walusinski, 2006). He
adds recently to his theory a more complete
explanation by involving the brain network that
is functional during the resting state, that is,
the default mode network. When this network is
active, yawning manifests a process of switching
to the attentional system through its capacity
to increase circulation of cerebrospinal fluid
(CSF), thereby increasing clearance of
somnogenic factors accumulating in the
cerebrospinal fluid (Walusinski, 2014).
-
- A further pro-arousal argument came from
Sato-Suzuki and co-workers who showed that
cortical EEG frequency - recorded experimentally
with 2 screws from the vertex of anesthetized
rats - increased prior to yawns elicited by
electrical or chemical stimulation of the PVN
and subsequently reversed to slow EEG activity
after yawning (Sato-Suzuki et al., 1998). These
behavioral and EEG phenomena could be repeated
with different stimuli such as orexins
(Sato-Suzuki et al., 2002) and histamine (Seki
et al., 2002). On the other hand, Guggisberg and
co- workers showed in 16 patients who suffered
from daytime sleepiness that yawning is
triggered by states of low vigilance but does
not lead to an arousing effect when compared to
the performance of voluntary isolated body
movements by the same group of patients. In
detail, yawning was preceded by significantly
greater delta activity in EEG than voluntary
movements, indicating drowsiness before the yawn
event. After yawning, power in the delta
frequency range was still significantly greater
than after a voluntary movement. In addition,
mean alpha frequency over central regions
increased after isolated movements while it
decreased after yawning indicating arousal
rather by voluntary body movements (Guggisberg
et al., 2007). Other studies investigating the
association of yawning with arousal in humans
with EEG, skin conductance, or measurements of
other autonomic parameters found controversial
results (Guggisberg et al., 2010, 2011). In
conclusion, yawning correlates with low states
of vigilance and is associated with transitions
between wake and sleep, periods of exposure to
uninteresting repetitive stimuli, or with
particular stressful events. The failure to
associate yawning with improved vigilance or
increased autonomic tone does not preclude
yawning to be a clinical sign for particular
activity states of those brain regions that are
involved in regulation or execution of yawning.
If this can be demonstrated in future
experiments, the arousal hypothesis may be
rephrased as focal brain activity
hypothesis.
-
- 4.4. Brain-cooling hypothesis
- One research group promoted brain cooling as
a biological function of yawning. Data of
arousal and autonomic activation in humans
(Corey et al., 2012) and transient brain
temperature peaks around bouts of yawning in
rats (Shoup-Knox et al., 2010) were presented as
evidence for this hypothesis. Abnormal yawning
was suggested to be indicative for
thermoregulatory dysfunction, i.e. disorders
temporarily associated with abnormal yawning
were associated with difficulties in body
temperature control (Gallup and Gallup, 2008;
Prasad, 2008; Gallup, 2014). It can be agreed
upon that (physiological) yawning and tongue
protrusion can help in the control of body
temperature in species such as felidae or
canines, which do not exchange heat as
effectively via body surface and perspiration as
do humans because of their fur. In humans, heat
exchange is likely to be more effective at the
body surface, promoted by perspiration, than in
lungs due to their surface difference. From a
neuroanatomical point of view the hypothala- mus
is believed to be the center for
thermoregulation, and the sympathetic nervous
system, which controls amongst others sweat
glands in skin, has origins in the hypothalamus,
as well. In addition, hypothalamic nuclei are
believed be one superordinate control system of
the yawning motor pattern. Therefore, we rather
suggest that neurological diseases or
neurotropic medication, affecting input/output
or function of the hypothalamus itself, might
conjointly affect thermoregulation and yawning
behavior. This does not necessarily mean that
these 2 mechanisms are causally related and one
is performed to serve the other. One needs to
take into consideration that a physiologist,
Hannu Elo, has shown by calculations that the
temperature decreases claimed to occur during
yawning are physically impossible (Elo,
2010).
-
- 5. Abnormal yawning associated with
diseases or medication
- Yawning may occur not only because of
boredom, drowsiness, or by contagion but also in
association with various pathologies. We tried
to give a representative review of diseases
associated with noticeable yawning, but may not
have covered all aspects. Abnormal yawning was
termed pathological or excessive in only a few
reports. For its definition including frequency,
the reader is referred to the introduction.
Several reports of noticeable yawning could
sometimes be better grouped according to a
neurological syndrome of various etiologies.
Otherwise, reports were listed according to the
respective neurological disease.
-
- 5.1. Yawning during and after
cerebrovascular insult
- 5.1.1. Anterior circulation
stroke
- Anterior circulation stroke with unilateral,
supratentorial lesions can be associated with
abnormal yawning. In the first report, 3 or more
yawns per 15 min were observed in each of 7
patients who presented with acute middle
cerebral artery stroke and signs of cortical
dysfunction such as aphasia, neglect and gaze
palsy. Symptom onset was within 12 h, average
NIHSS score was 17 and 1/3 or more of the MCA
territory was affected. The authors' conclusion
was that supratentorial lesions may release the
hypothalamic PVN from neocortical control
mechanisms, thereby increasing its activity and
leading to excessive yawning. As temporal lobe
structures were partially damaged in their
patients, they speculated that
hippocampal/periamygdalar - hypothalamic
connections, shown before to exist, might have
been affected (Singer et al., 2007). The second
report was about a patient with left internal
capsule stroke and right-sided weakness (Upper
and lower limb 0/5 and 2/5 respectively), who
could not move his arm voluntarily but was able
to do so during yawning. The authors speculated
that intact non-pyramidal projections from a
putative, non-lesioned yawning center in the
brainstem might share the common lower motor
neuron pathway to innervate brainstem nuclei and
spinal cord anterior horn cells. It was not
mentioned whether the patient exhibited
excessive yawning (Wimalaratna and Capildeo,
1988). Involuntary yawning-associated movements
in paralyzed limbs were termed parakinesia
brachialis oscitans (Walusinski et al., 2005).
They were also observed in a large case series
of patients with acute anterior circulation
stroke. Interest- ingly, these
yawning-associated movements do not seem to have
any prognostic value (Meenakshisundaram et al.,
2010). We published a series of 10 patients with
acute anterior circulation stroke and !3
yawns/15 min without obvious cause. We showed
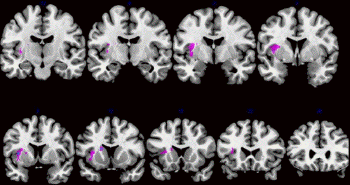
that the strongest lesion overlap was in the
insula and caudate nucleus (Fig. 4) and that
duration of abnormal yawning correlated with
clinical stroke severity (NIHSS) and with
neuroradiological stroke intensity (inverse
apparent diffusion coefficient correlation) in
the strategically lesioned brain areas (Krestel
et al., 2015). Based on the insula's intense
brainstem connections via the cortico-
striato-thalamic network and corticobulbar
pathways, we sug- gested following mechanisms
upon its lesioning: i) a disconnection syndrome
of insular targets including the entorhinal
cortex, lateral hypothalamus, or
mono-/oligosynaptic trajectories to the Raphe
nucleus/nucleus tractus solitarius with
(GABA-ergic?) disinhibi- tion of the nearby
pre-Bötzinger complex respiratory rhythm
generator and cranial nerve nuclei V, VII, IX,
X, and XII. ii) A serotonin overspill theory
upon partial insular lesioning with activation
of the brainstem yawning pattern in analogy to
experimental infusion of serotonin agonists into
the insula with gaping in awake rats (Tuerke et
al., 2012). Anterior circulation stroke with
bilateral supratentorial lesions - termed
bilateral anterior opercular syndrome or
Foix-Chavany-Marie Syndrome - was associated in
several reports with yawning despite paralysis
of voluntary facial and pharyngeal innervation.
Neuroanatomically, a bilateral lesion of the
fronto-parietal operculum is found and an
impairment of the proximal corticobulbar tract
is implicated. This syndrome arises in its
classical and most common form due to
cerebrovascular disease, but other causes are
also possible such as CNS infections,
developmental deficits, and rarely neurodegener-
ative processes. A reversible form after
epileptic seizures is also described (Ghika et
al., 2003; Millán et al., 2008; Billeth
et al., 2000; Laurent-Vannier et al.,
1999).
-
- 5.1.2. Posterior circulation
stroke
- Posterior circulation insults with
infratentorial vascular lesions have also been
associated with abnormal yawning. In one report,
two patients presented with excessive yawning
(0.5-3 per min) less than an hour before onset
of neurological deficits, consisting of gait
ataxia in both cases with additional
brachio-facial hemiparesis and internuclear
ophthalmoplegiea in the second patient. The
first patient had a lacunar paramedian pontine
infarction. His yawning frequency decreased
rapidly after the insult and disappeared after 3
days together with his neurological deficits.
The second patient had an infarction at the
ponto-mesencephalic junction due to
pseudo-occlusive stenosis of the basilar artery.
Her yawning ceased after 1.5 days while deficits
persisted (Cattaneo et al., 2006). The authors
suggest a denvervation hypersensitivity
mechanism, consisting of liberation of a
putative yawning center in the brainstem from
[inhibitory] control of more cranial
structures, in analogy to theories about
excessive yawning in ALS patients (Williams,
2000) or in a case with hiccups after medullary
infarction (Park et al., 2005).
-
- 5.2. Locked-in syndromes and
yawning
- 5.2.1. Locked-in syndrome in vascular
pathology of the brain
- Locked-in syndromes have been associated
with yawning. Their etiologies are mainly
ischemic brainstem lesions. Accordingly,
following reports were found in the literature:
Nordgren et al., 1971; Karp and Hurtig, 1974;
Krasnianski et al., 2003; Chang et al., 2008. In
one report, the origin of the locked-in
syndromes was not explicitly mentioned but
assumed to be vascular because of acute onset
and regression of symptoms (Bauer et al.,
1980).
-
- 5.2.2. Locked-in syndrome caused by
tumor
- Yawning has also been reported in locked-in
syndrome due to a brainstem tumor (Gschwend,
1977). Here, a patient was admitted to the
Neurological University Hospital Berne with
tetraplegia including complete voluntary
paralysis of all muscles innervated by caudal
brainstem nerves. Hence, he could not open his
mouth but was able to yawn. Remarkably, the
patient could not imitate when somebody yawned
in front of him, in other words contagious
yawning was not possible. Postmortem studies
showed infiltration of ventral pons with
destruction of the pyramidal tract and caudal
extention to the cerebello-pontine angle.
Proximal to the tumor, the oculomotor and
trochlear nuclei were intact and therefore the
patient could move his eyes. The author
concluded that yawning was "motivated" by a
center below the pontine lesion and proposed the
medulla oblongata and more precisely the Raphe
nuclei. This suggestion was traced back on
experiments that electrical stimulation of raphe
nuclei could elicit sleep in cats (Jouvet et
al., 1967). Gschwend associated yawning in his
patient with sleepiness, because contagious
yawning, implying higher levels of vigilance,
was not possible. Therefore, he also cited work
about induction of sleepiness by diencephalic
electrical stimula- tion (Hess, 1944) and
suggested its effect to be mediated by raphe
nuclei, as well. However, sleepiness in cats was
in none of the publications (Jouvet, Hess)
reported to be accompanied by yawning. Lack of
contagious yawning with preservation of
spontaneous yawning in Gschwend's patient may be
caused by disruption of several control pathways
[control of a putative yawning center by the
pyramidal tract, hypothalamic, and extra-
hypothalamic efferents (see 6. Conclusions and
perspectives)] leaving a brainstem network
akin to its own irregular activity to elicit
yawning from time to time.
-
- 5.3. Brain tumors
- Another report of a brainstem tumor
associated with sponta- neous yawning was a
patient with an hemangioblastoma, verified by
biopsy, that was located in ["the lower hall
of"] the 4th ventricle and caused autonomic
symptoms such as orthostatic hypotension but
also spontaneous yawning probably by increased
intracra- nial pressure (Arai et al., 1986).
Brain tumors as a cause of yawning were also
recognized earlier: "the occurrence of
[excessive] yawning which is essentially
an atypical respiratory act, in cases of brain
tumor can be explained by irritation of the
brain resulting in afferent impulses to the
medullary respiratory center which modifies its
rhythmic activity" (Nash, 1942).
-
- 5.4. Traumatic brain injury
- Yawning was mentioned in the context of
traumatic brain injury in the following reports.
Coma outcome was assessed on the basis of MRI
lesion load and presence of clinical sings such
as yawning, grasping and chewing in mechanically
ventilated patients after cessation of sedation
(Weiss et al., 2008). In another work, it was
suggested that yawning during hyperventilation
in awake patients after recent head injury may
serve as a sign of brain damage, especially at
the brainstem level (Jurko and Andy, 1975).
-
- 5.5. Persistent vegetative state
- Patients with persistent vegetative state of
different etiologies were also reported to yawn
spontaneously. Six months post resuscitation of
cardiac arrest, the patient with hypoxic
encepha- lopathy was in a persistent vegetative
state being awake and showing spontaneous eye
movements, chewing and yawning but no purposeful
movement on verbal or visual stimuli (Manish and
Veenu, 2007). Yawning was mentioned as one of
several symptoms in persistent vegetative state
due to traumatic etiology in a German monography
(Gerstenbrand, 1967). Intracranial hypertension,
whether related to stroke, tumor, or head trauma
can be revealed by headaches, impaired
vigilance, seizures, and be associated with
salvos of yawns. Certain coma scores used in USA
take into account the presence of yawning in
these situations (Bateman, 2001).
-
- 5.6. Epilepsy
- An association of yawning with epilepsy has
been described in case reports and case series
as peri-ictal yawning. Many reports are based on
temporary coincidence of symptoms, statistical
associ- ations or successful diagnostic
treatment trials, respectively. One early
publication contained two detailed reports that
were associated with epilepsy according to their
symptoms. The first patient suffered from
occipital headache, compulsive yawning or
singultus, accompanied by a reversible sensory
cheiro-oral syndrome. The second was a young man
with sudden sweating, goose-skin, derealisation
symptoms and compulsive yawning. (Penfield and
Jasper, 1954). In a later report, 3 children
with absence epilepsy underwent long term EEG
recording. In one of three, a 7-year old child,
yawning was significantly more prevalent in pre-
and ictal periods compared to the rest of the
EEG recording (Goldie and Green, 1961).
Subsequently, a patient was reported with
attacks of occipital headache and compulsive
yawning. Laboratory analyses and cerebral CT
were normal. A surface EEG recording showed
slight irregularities in occipital channels.
However, using sphenoidal electrodes, a
generalized paroxysmal pattern was described
during one of the patient's attacks.
Carbamazepine reduced the frequency of yawning
attacks and naloxone, an opioid antagonist,
could completely suppress them (Fletcher et al.,
1982). The Foix-Chavany-Marie syndrome is again
mentioned here (see also 5.1.1. Anterior
circulation stroke) because a reversible form
was described to exist in epilepsy. It was
delineated as a deficit of voluntary control of
muscles innervated by nerves V, VI, IX, X, XI
with dissociation of automatic patterns such as
laughing, crying, yawning, which were still
possible (Laurent-Vannier et al., 1999).
-
- Two more case reports of yawning and
potential epileptic seizures were presented by
Muchnik et al. A single episode of a compulsive
yawning attack, followed by loss of contact with
fixed gaze for 30s and subsequent confusion and
orthograde amnesia was reported in a 95-year old
male patient. Cerebral CT showed general
cerebral atrophy and bitemporal, irritative EEG
activity was found. Carbamazepine 400mg/d
prevented any recurrence of yawning. The second
case was a 17-year old woman with
insulin-dependent diabetes and repeti- tive
reports of hypoglycemic fainting. Once, she
fainted with generalized tonic-clonic
convulsions, followed by confusion and
compulsive yawning. Valproate was successful in
preventing future fainting with convulsions
(Muchnik et al., 2003). Then a 61-year old woman
with known migraine and bipolar disorder was
reported who suffered from attacks of ascending
epigastric sensation, compulsive yawning for 30
min, diffuse sweating and subsequent sleepiness
several times daily since 2 years. Such an
attack could be elicited by photostimulation
during an EEG recording that did not show
epileptic potentials. However, during subsequent
sleep, steep potentials were noted left
temporal. After awakening the patient did not
remember her attack. Oxcarbama- zepine 600 mg/d
could suppress recurrence of these attacks
within 2 weeks (Medrano et al., 2005). Finally,
a 47-year old woman was described, who suffered
from treatment-resistant epileptic seiz- ures of
various semiologies for many years. In recent
years, especially following posturing-type
seizures, postictal compulsive yawning is noted.
This publication also contains a literature
review of epilepsy and yawning (Yankovsky et
al., 2006). Peri-ictal yawning was evaluated as
lateralizing sign in 97 patients with temporal
lobe seizures and was found to occur only in the
postictal period after seizures in the
non-dominant, right-sided hemisphere (Kuba et
al., 2010). Of note is that gelastic seizures,
frequently arising from hypothalamic hamartoma
with smiling as typical semiological sign of a
seizure, have not yet been described to be
associated with yawning. Conceivably, a tumor in
the PVN's vicinity might activate (by ictal
spread) or deafferentiate the PVN and lead to
abnormal yawning, but this has not yet been
reported in the literature.
-
- 5.7. Migraine
- Yawning is one of the premonitory symptoms
in migraine. Although the brainstem is highly
linked to migraine biology, the real driver of
attacks might be functional changes in
hypothalamic- brainstem connectivity, explaining
typical migraine premonitory symptoms such as
fatigue and yawning, but also a typical
association of attacks to circadian and
menstrual cycles, all making the hypothalamus a
possible regulating region of migraine attacks
(Laurell et al., 2016). Revitalized interest in
dopamine as important transmitter in migraine is
based, first, on the observa- tion that
premonitory symptoms such as yawning,
drowsiness, mood changes, irritability, and
hyperactivity are exacerbated by dopamine
agonists. Second, D1 and D2 dopamine receptors
were found in the trigeminocervical complex.
Third, dopaminergic projections from the
hypothalamus to the trigeminocervical complex
and further to the spinal cord exist (reviewed
in Akerman and Goadsby, 2007). Based on the
analogy that dopamine agonists cause yawning,
nausea & blood pressure changes that are
similar to some of the premonitory symptoms
found in migraine, the dopamine theory of
migraine was proposed, originally by Sicuteri
(1977). It says that a migrainous brain is
hypersensitive to dopamine and subsequent
genetic studies implied that polymor- phisms in
dopamine receptor genes may create this
hypersensi- tivity. Due to this
hypersensitivity, dopamine may be one of the
triggers that drive a migraine attack. Support
for this theory comes from above mentioned
neuroanatomical findings and from empirical
treatment. Domperidone (20/30mg), a peripheral
D2 receptor antagonist, decreased the duration
of an attack by 30%, but only when combined with
paracetamol (MacGregor et al., 1993).
-
- Domperidone/paracetamol has also been shown
to be similarly efficacious as sumatriptan
(50mg) over a 2- and 4-h postdose period (Dowson
et al., 2000). One explanation for these results
is that dopamine antagonists prevent the nausea
accom- panying the attack, and then correct the
possible hypersensitivity to dopamine in the
brain. However, data about involvement of
dopamine in migraine pain is less clear than its
involvement in premonitory symptoms. For
example, dopamine agonists were shown to inhibit
neuronal firing in the trigeminocervical
complex, but, as mentioned above, it is dopamine
antagonists that are clinically effective
(Akerman and Goadsby, 2007). Of note is also the
following study that migraine patients with
restless legs syndrome (RLS) have significantly
more frequent (5 ) migraine premonitory symptoms
including yawning than migraine patients without
RLS. The authors conclude that RLS is more
common in migraine with premonitory symptoms and
suggest a common imbalance of the dopaminergic
system in RLS and migraine (Cologno et al.,
2008). Functional imaging using positron
emission tomography and later fMRI of a migraine
patient every day for 30 days in the morning
covered 3 complete, untreated migraine attacks
and showed that hypothalamic activity increased
towards the next migraine attack during the 24 h
prior to pain onset. The hypothalamus also
showed altered functional coupling with the
spinal trigeminal nuclei and the dorsal rostral
pons during the pre- ictal day and the pain
phase of the native migraine attack, suggesting
that the hypothalamus might be a migraine
generator and be responsible for premonitory
symptoms such as fatigue and yawning, but can
also explain a typical association of attacks to
circadian and menstrual cycles. (Schulte and
May, 2016).
-
- 5.8. Neurodegenerative disease
- 5.8.1. Amyotrophic lateral
sclerosis
- Excessive yawning has also been reported in
neurodegenerative diseases. We would like to
begin this section with notes of patients with
motor neuron disease or amyotrophic lateral
sclerosis (ALS). A 64-year old woman was
referred to the doctor with paroxysmal bouts of
compulsive yawns occurring 20-30 times in
succession seven to ten times a day. Initial
neurological examination was normal. Yawning
could be suppressed with thioridazine an
antagonist to dopamine D2, histamine H1,
alpha-adrenergic and other receptors. In the
course of nine months, the patient developed
dysphagia with bulbar and pseudobulbar clinical
signs. Thioridazine was suspended and bouts of
yawning reoccurred. Electrophysiological
examination showed signs of denervation in all 4
extremities and diagnosis of motor neuron
disease was made. Her yawning became less
evident as her bulbar palsy progressed.
Theauthor'sinterpretationwasthatyawningmaybeinhibitedin
mature humans and the responsible brainstem
neurons must be subject to cortical influences
that can only be expressed if upper motor neuron
pathways are intact. In pseudobulbar palsy,
inhibition by upper motor neurons is lost and
spontaneous yawning is released. Yawning will
disappear with significant malfunction of bulbar
nuclei. Therefore, yawning may be a subtle sign
of pseudobulbar palsy (Williams, 2000). The next
report refers to 254 patients with ALS who
completed a questionnaire on excessive yawning
at an internet website. Yawning was reported to
be absent in 30%, mild in 30%, moderate in 32%
and severe in 9%. No correlation was found
between severity of yawning and age, months
since diagnosis or the last recorded measurement
of forced vital capacity. There was no
association between yawning severity and
anti-depressant usage. However, there was an
association between yawning severity and site of
onset. Patients with bulbar onset of disease
were more likely (57%) to have moderate or
severe yawning than patients with arm onset
(42%) or leg onset (31%) (Wicks, 2007). Wicks'
observation is supported by the case report of
Williams in which the patient had bulbar onset
of motor neuron disease, as well. Finally, an
article about ALS and yawning by Louwerse ES et
al. J Neurosci Res 1998 is occasionally
mentioned. However, the article may be not
correctly cited as it cannot be found in PubMed
or on the homepage of the Journal of
Neuroscience Research itself.
-
- 5.8.2. Yawning in untreated
extrapyramidal disease
- Abnormal yawning has also been reported in
extrapyramidal disease. We would like to regroup
these reports into untreated versus treated
hypokinetic movement disorders, i.e. in which
abnormal yawning is inherent in the condition or
medically promoted. In the untreated condition,
the earliest reports about yawning are in the
acute encephalitic stage of encephalitis
lethargica but also in post encephalitic
parkinsonian patients (Sicard and Paraf 1921;
Mayer, 1921). This was also reported by von
Economo (Wimmer, 1924; mentioned in Colosimo and
Pontieri, 1999, and reviewed in Evidente et al.,
1998). Despite these reports, compulsive or even
abnormal yawning is not that frequent in
untreated idiopathic [non-encephalitic]
Parkinson's disease (PD) patients. In fact, in
one publication it was even stated that yawning
has not been associated with PD (Goren and
Friedman, 1998). This report was followed by
several comments including the above mentioned
by Colosimo and Pontieri (1999), then by
Evidente and Hardy and O'Sullivan et al. which
all were published in the same issue of
Neurology, 15 1999, pointing to encephalitis
lethargica with its sequelae and to side effects
of dopaminergic therapy.
-
- 5.8.3. Yawning in treated extrapyramidal
disease
- Apart from PD, there is a report about five
untreated patients with progressive supranuclear
palsy, an atypical Parkinson's syndrome with
vertical gaze paralysis and frequent drops, who
all exhibited abnormal yawning (1-3 yawns/min).
Yawning subsided with administration of
dopaminergic medication (L- DOPA + carbidopa or
bromocriptin, a D2/D3 receptor agonist; Sandyk,
1987). It is surprising that dopaminergic
medication decreased yawning frequency instead
of increasing it according to neurochemical and
clinical observations (see neurochemistry
section above). On the other hand, dopaminergic
medication in PD was associated with yawning.
Yawning was heralded as early sign for L-DOPA
induced ON in PD (Goren and Friedman, 1998).
Yawning may also appear while using apomorphine
to diagnose dopamine responsiveness or to rescue
OFF states in advanced PD patients (Ferraz et
al., 1995; Frankel et al., 1990; Bonuccelli et
al., 1991; Colosimo et al., 1994; Pahwa et al.,
2007). Again, yawning followed subcutaneous
apomorphine application in an early double blind
placebo controlled study for parkinsonian OFF
events. (Dewey et al., 2001) and in a meta
review about clinical studies that use
apomorphine s.c. to rescue OFF states in
advanced PD patients under optimal dopaminergic
therapy (Chen and Obering, 2005). Side effects
of subcutaneous apomorphine for OFF events
despite optimized oral medication in 546 PD
patients included dyskinesia, somnolence,
hallucina- tion, yawning and injection site
bruising and prevented 36% of patients from
continuing apomorphine injections (LeWitt et
al., 2009).
-
- 5.9. Multiple sclerosis
- Multiple Sclerosis (MS) could also lead to
abnormal yawning, as reported in a patient with
left spastic hemiparesis and yawning 4 /min,
despite regular and long sleep, which remitted
after steroid infusion. MRI showed multiple
brainstem lesions [supra- tentorial lesions
were not mentioned]. The authors concluded
that brainstem plaques irritated reticular
neurons including the RAS and caused yawning.
These "irritated" cells (ephaptic coupling?
Annotation by this review's authors) send their
impulses via intact short interconnections to
motor nuclei of cranial nerves involved in
yawning (Postert et al., 1996). Abnormal yawning
appeared during a relapse in another patient
with relapsing-remitting MS concomitantly with
new T2-hyperintense, neuroradiological lesions
in supratentorial brain including the left
hippocampus and caudate nucleus, and the frontal
commissure (Fig. 5; unpub- lished
observation).
-
- 6. Conclusion and perspectives
- A number of important systems and circuits
exist in the evolutionary old sector of the
human brainstem that are not modifiable by
experience and are shared among numerous other
species. The innate activity patterns of neurons
in these circuits are not subject to mind or
reason and frequently regulate basic life
processes including breathing, coughing, or
swallowing. Physio- logical yawning takes an
intermediate position because it is under
partial voluntary control and is switched off
during sleep while other basic patterns are not.
Several concepts exist concerning the mechanism
of yawning. An integration and extension of the
networks and brain regions involved in yawning
is proposed. The neocortex, limbic system, and
thalamus may be involved in external pattern
recognition and may be responsible for the
contagiousness of yawning, be it via the
neuronal system of imitation (mirror neurons),
or the system integrating empathy (theory of
mind). The yawning network or zone is suggested
to consist of parts of cortex, limbic system,
hypothalamus, and brainstem. From anatomical
malformations and lesion studies there is little
doubt that the core yawning center resides in
the brainstem. Its proposed functions beyond
arousal suggest that the yawning center is not
in the vicinity of the RAS with its most caudal
circuits in the midbrain reticular formation and
in mesencephalic and pontine nuclei, but rather
located in the ponto-medullary part of the
brainstem near the central pattern generators
for respiration and circulation and the motor
nuclei of cranial nerves V, VII, IX, XI,
implicated in the yawning act.
-
- Probably 3 supratentorial pathways fine-tune
the innate activity of the brainstem yawning
center. Impairment or disconnection of
corticobulbar trajectories could explain
observed abnormal yawning in Foix-Chavany-Marie
syndrome, locked-in syndrome, and bulbar ALS. It
remains to be shown whether pyramidal
corticobulbar trajectories are part of the
yawning network or if thalamo-bulbar motor
trajectories are also involved. They run more
dorsally in the brainstem close to the central
gray and are affected e.g. in emotional facial
paralysis. Electrical and chemical stimulation
of the PVN with its hypothal- amo-pontomedullary
trajectories has been shown to affect yawning.
Of note is that hypothalamic brain tumors have
not been particularly associated with abnormal
yawning, nor does yawning typically accompany
gelastic seizures that frequently arise from the
hypothalamus due to hypothalamic hamartoma. The
insula as part of the telencephalic cortex may
be a brain region for serotonin-mediated
yawning. Inclusion of the insula into the
yawning network may explain why peri-ictal
yawning is frequently seen in temporal lobe
epilepsy in which seizure activity may also
spread to the insula. A partial loss of function
of the insula and its trajectories to the
entorhinal cortex, lateral hypothalamus, or
mono-/oligosynaptic trajectories to the Raphe
nucleus/nucleus tractus solitarius by e.g.
stroke or demyelinating lesions may affect
abnormal yawning, as well.
-
- All theories of a top-down control of a
brainstem yawning center imply an
inhibition-disinhibition concept. Except of the
PVN with oxytocin and NO as transmitter at its
brainstem synapses, the disinhibition theories
in abnormal yawning suggest a dysfunction or
loss of GABA-ergic synapses onto the putative
brainstem yawning center. Although the
neurochem- istry at the level of the
supratentorial control centers may be fairly
well characterized, i.e. which transmitters and
molecules elicit or inhibit yawning at the brain
rostral to the brainstem, the neurochemistry and
synaptic connections at the ponto-medullary
brainstem, that is involved in yawning, is not.
In order to advance the knowledge on this
ancient, stereotype behavior; and shed light on
other reflex-like behaviors including coughing,
swallowing; the neuroanatomy and - chemistry of
yawning at the level of the brainstem has to be
much more better understood. Not only the motor
pattern but probably also yawning's meaning is
preserved across species barriers, i.e. also the
observer of yawning from another species may
have a similar understanding of what yawning
signals. The role of yawning and what it conveys
may be to express sleepiness and arousal,
indicate boredom and wellbeing and further
messages and functions. The body of reports of
abnormal yawning is encompassing. It probably
already reflects most of the etiologies of
abnormal yawning. Taken together, physiological
yawning occurs activity-dependent within intact
circuits. Abnormal yawning arises from lesions
of brain areas involved in the yawning zone, its
trajectories causing a disconnec- tion syndrome,
or from physical or metabolic alteration of
network activity. An important perspective would
be a better understand- ing of yawning's
neuroanatomy and - chemistry at the brainstem
level.
-
- References
- Abdala, A.P., Rybak, I.A., Smith, J.C.,
Zoccal, D.B., Machado, B.H., St-John, W.M.,
Paton, J.F., 2009. Multiple pontomedullary
mechanisms of respiratory rhythmogenesis.
Respir. Physiol. Neurobiol. 168, 19-25.
-
- Akerman, S., Goadsby, P.J., 2007. Dopamine
and migraine: biology and clinical implications.
Cephalalgia 27, 1308-1314.
-
- Anderson, J.R., Wunderlich, D., 1988.
Food-reinforced yawning in Macaca tonkeana.
Amer. J. Primatol. 16, 165-169.
-
- Arai, K., Kita, K., Komiyama, A., Hirayama,
K., Saeki, N., Nagao, K., 1986. Progressive
dysautonomia in hemangioblastoma in the region
of the fourth ventricle. No To Shinkei 38,
195-200 (Article in Japanese).
-
- Argiolas, A., Gessa, G.L., 1991. Central
functions of oxytocin. Neurosci. Biobehav. Rev.
15, 217-231.
-
- Argiolas, A., Melis, M.R., 1998. The
neuropharmacology of yawning. Eur. J. Pharmacol.
343, 1-16.
-
- Argiolas, A., Melis, M.R., Murgia, S.,
Schiöth, H.B., 2000. ACTH- and alpha-MSH-
induced grooming stretching, yawning and penile
erection in male rats: site of action in the
brain and role of melanocortin receptors. Brain
Res. Bull. 51, 425- 431.
-
- Arnott, S.R., Singhal, A., Goodale, M.A.,
2009. An investigation of auditory contagious
yawning. Cogn. Affect Behav. Neurosci. 9
(September (3)), 335-342.
-
- Askenasy, J.J., 1989. Is yawning an arousal
defense reflex? J. Psychol. 123, 609-621.
Baenninger, R., Greco, M., 1991. Some
antecedents and consequences of yawning.
Psychol. Rec. 41, 453-460.
-
- Baenninger, R., Binkley, S., Baenninger, M.,
1996. Field observations of yawning and activity
in humans. Physiol. Behav. 59, 421-425.
-
- Baenninger, R., 1997. On yawning and its
functions. Psychon. Bull. Rev. 4, 198-207.
Baladi, M.G., Newman, A.H., France, C.P., 2010.
Dopamine D3 receptors mediate the discriminative
stimulus effects of quinpirole in free-feeding
rats. J. Pharmacol.Exp. Ther. 332, 308-315.
-
- Bateman, D.E., 2001. Neurological assessment
of coma. J. Neurol. Neurosurg. Psychiatry 71,
111-117.
-
- Bauer, G., Gerstenbrand, F., Hengl, W.,
1980. Involuntary motor phenomena in the
locked-in syndrome. J. Neurol. 223,
191-198.
-
- Bechara, A., Damasio, H., Damasio, A.R.,
Lee, G.P., 1999. Different contributions of the
human amygdala and ventromedial prefrontal
cortex to decision-making. J. Neurosci. 19,
5473-5481.
-
- Bechara, A., Tranel, D., Damasio, H., 2000.
Characterization of the decision-making deficit
of patients with ventromedial prefrontal cortex
lesions. Brain 123, 2189- 2202.
-
- Bell, L., 1980. Boredom and the yawn. Rev.
Existent. Psychol. Psychiatry 17, 91-100.
-
- Beltramo, M., de Fonseca, F.R., Navarro, M.,
Calignano, A., Gorriti, M.A.,Grammatikopoulos,
G., Sadile, A.G., Giuffrida, A., Piomelli, D.,
2000. Reversal of dopamine D(2) receptor
responses by an anandamide transport inhibitor.
J. Neurosci. 20, 3401-3407.
-
- Billeth, R., Jörgler, E., Baumhackl,
U., 2000. Bilateral anterior operculum syndrome.
Nervenarzt 71, 651-654.
-
- Bonuccelli, U., Piccini, P., Del Dotto, P.,
Rossi, G., Corsini, G.U., Muratorio, A., 1991.
Naloxone partly counteracts apomorphine side
effects. Clin. Neuropharmacol. 14, 442-449.
-
- Brown, R.W., Perna, M.K., Schaefer, T.L.,
Williams, M.T., 2006. The effects of adulthood
nicotine treatment on D2-mediated behavior and
neurotrophins of rats neonatally treated with
quinpirole. Synapse 59, 253-259.
-
- Buijs, R.M., Swaab, D.F., Dogterom, J., van
Leeuwen, F.W., 1978. Intra- and
extrahypothalamic vasopressin and oxytocin
pathways in the rat. Cell Tissue Res. 186,
423-433.
-
- Buijs, R.M., Geffard, M., Pool, C.W.,
Hoorneman, E.M., 1984. The dopaminergic
innervation of the supraoptic and
paraventricular nucleus: a light and electron
microscopical study. Brain Res. 323, 65-72.
-
- Buijs, R.M., 1978. Intra- and
extrahypothalamic vasopressin and oxytocin
pathways in the rat. Pathways to the limbic
system, medulla oblongata and spinal cord. Cell
Tissue Res. 192, 423-435.
-
- Catel, W., Krauspe, C.A., 1930. Über
die nervöse Leistung und den anatomischen
Bau einer menschlichen Hirnmissbildung
(Meroanenzephalie mit Meroakranie). Jahrb. f.
Kinderh. 129, 1.
-
- Cattaneo, L., Cucurachi, L., Chierici, E.,
Pavesi, G., 2006. Pathological yawning as a
presenting symptom of brain stem ischaemia in
two patients. J. Neurol. Neurosurg. Psychiatry
77, 98-100.
-
- Chang, C.C., Chang, S.T., Chang, H.Y., Tsai,
K.C., 2008. Amelioration of pathological yawning
after tracheostomy in a patient with locked-in
syndrome. Eur. J. Neurol. 15, e66-e67
-
- Chen, C.H., Lu, M.H., 2009.
Venlafaxine-induced excessive yawning: prog.
Neuropsychopharmacol. Biol. Psychiatry 33,
156-157.
-
- Chen, J.J., Obering, C., 2005. A review of
intermittent subcutaneous apomorphine injections
for the rescue management of motor fluctuations
associated with advanced Parkinson's disease.
Clin. Ther. 27, 1710-1724.
-
- Collins, G.T., Eguibar, J.R., 2010.
Neuropharmacology of yawning. In: Walusinski, O.
(Ed.), The Mystery of Yawning in Physiology and
Disease. Front. Neurol. Neurosci. Karger,
Basel.
-
- Collins, G.T., Woods, J.H., 2008. Narrowing
in on compulsions: dopamine receptor functions.
Exp. Clin. Psychopharmacol. 16, 498-502.
-
- Collins, G.T., Calinski, D.M., Newman, A.H.,
Grundt, P., Woods, J.H., 2008. Food restriction
alters
N'-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine
dihydrochloride (pramipexole)-induced yawning,
hypothermia, and locomotor activity in rats:
evidence for sensitization of dopamine D2
receptor-mediated effects. J. Pharmacol. Exp.
Ther. 325, 691-697.
-
- Cologno, D., Cicarelli, G., Petretta, V.,
d'Onofrio, F., Bussone, G., 2008. High
prevalence of Dopaminergic Premonitory Symptoms
in migraine patients with Restless Legs
Syndrome: a pathogenetic link? Neurol. Sci. 29
(Suppl 1), S166- S168.
-
- Colosimo, C., Pontieri, F.E., 1999. Yawning
in Parkinson's disease. Neurology 52, 428.
Colosimo, C., Merello, M., Albanese, A., 1994.
Clinical usefulness of apomorphine in movement
disorders. Clin. Neuropharmacol. 17,
243-259.
-
- Corey, T.P., Shoup-Knox, M.L., Gordis, E.B.,
Gallup Jr., G.G., 2012. Changes in Physiology
before, during, and after Yawning. Front. Evol.
Neurosci. 3, 7.
-
- Coupar, I.M., Tran, B.L., 2001. Withdrawal
and bidirectional cross-withdrawal responses in
rats treated with adenosine agonists and
morphine. Life Sci. 69, 779-790.
-
- Daquin, O., Micallef, J., Blin, O., 2001.
Yawning. Sleep Med. Rev. 5, 299-322.
-
- de Gorter, J., 1725. De Perspiratione
Insensibili Sanctoriana-Batava: Tractatus
Experimentis Proprii In Hollandia. Sumptibus
Auctoris & Prostant Janssonois Vander,
Lugduni Batavorum (246 p).
-
- De Las Cuevas, C., Sanz, E.J., 2007.
Duloxetine-induced excessive disturbing and
disabling yawning. J. Clin. Psychopharmacol. 27,
106-107.
-
- Depoortère, R., Bardin, L.,
Rodrigues, M., Abrial, E., Aliaga, M.,
Newman-Tancredi, A., 2009. Penile erection and
yawning induced by dopamine D2-like receptor
agonists in rats: influence of strain and
contribution of dopamine D2, but not D3 and D4
receptors. Behav. Pharmacol. 20, 303-311.
-
- Dewey Jr., R.B., Hutton, J.T., LeWit, P.A.,
Factor, S.A., 2001. A randomized, double- blind,
placebo-controlled trial of subcutaneously
injected apomorphine for parkinsonian off-state
events. Arch. Neurol. 58, 1385-1392.
-
- Doger, E., Urbá-Holmgren, R.,
Eguibar, J.R., Holmgren, B., 1989. GABAergic
modulation of yawning behavior. Pharmacol.
Biochem. Behav. 34, 237-240.
-
- Dowson, A., Ball, K., Haworth, D., 2000.
Comparison of a fixed combination of domperidone
and paracetamol (Domperamol) with sumatriptan 50
mg in moderate to severe migraine: a randomised
UK primary care study. Curr. Med. Res. Opin. 16,
190-197.
-
- Dumpert, V., 1921. Zur kenntnis des wesens
und der physiologischen bedeutung des
Gähnens. J. Psychol. Neurol. 27, 82.
-
- Duus, P., 1995. Neurologisch-topische
Diagnostik. 6. überarbeitete Auflage. Georg
Thieme Verlag, Stuttgart.
-
- Elo, H., 2010. Yawning and thermoregulation.
Sleep Breath. 14, 391-392.
-
- Endoh, T., 2007. Muscarinic M2 receptor
inhibition of calcium current in rat nucleus
tractus solitarius. Neuroreport 18,
1141-1145.
-
- Eslinger, P.J., 1998. Neurological and
neuropsychological bases of empathy. Europ.
Neurol. 39, 193-199.
-
- Evidente, V.G., Hardy, K.G., 1999. Yawning
in Parkinson's disease. Neurology 52, 428.
-
- Evidente, V.G., Gwinn, K.A., Caviness, J.N.,
Muenter, M., Mulder, D.W., 1998. Early
cinematographic cases of postencephalitic
parkinsonism and other movement disorders. Mov.
Disord. 13, 167-169.
-
- Fellows, L.K., Farah, M.J., 2007. The role
of ventromedial prefrontal cortex in decision
making: judgment under uncertainty or judgment
per se? Cereb. Cortex 17, 2669-2674.
-
- Ferraz, H.B., Azevedo-Silva, S.M., Borges,
V., Rocha, M.S., Andrade, L.A., 1995.
Apomorphine: an alternative in the control of
motor fluctuations in Parkinson's disease. Arq.
Neuropsiquiatr. 53, 245-251
-
- Fletcher, S., Cohen, F., Borenstein, F.,
Regev, I., Vardi, J., 1982. Yawning as a
paroxysmal sign of diencephalic seizures. Arch.
Psychol. Psychiatry Neurol. 43, 45-54.
-
- Frankel, J.P., Lees, A.J., Kempster, P.A.,
Stern, G.M., 1990. Subcutaneous apomorphine in
the treatment of Parkinson's disease. J. Neurol.
Neurosurg. Psychiatry 53, 96-101.
-
- Freye, E., Levy, J., 2005. Constitutive
opioid receptor activation: a prerequisite
mechanism involved in acute opioid withdrawal.
Addict. Biol. 10, 131-137.
-
- Gallup, A.C., Gallup Jr., G.G., 2008.
Yawning and thermoregulation. Physiol. Behav.
95, 10-16.
-
- Gallup, A.C., 2014. Abnormal yawning in
stroke patients: the role of brain
thermoregulation. Front. Neurosci. 8, 300.
-
- Gamper, E., 1926. Bau und leistungen eines
menschlichen mittelhirnwesens (Arhinencephalie
mit encephalocele) zugleich ein beitrag zur
teratologie und fasersystematik. Ztschr. Neurol.
Psychiat. 104, 49.
-
- Gerstenbrand, F., 1967. Das Apallische
Syndrom. Wien, Springer, New York. Ghika, J.,
Vingerhoets, F., Bogousslavsky, J., 2003.
Dissociated preservation of automatic-voluntary
jaw movements in a patient with biopercular and
unilateral pontine infarcts. Eur. Neurol. 50,
185-188.
-
- Goessler, U.R., Hein, G., Sadick, H.,
Maurer, J.T., Hörmann, K., Verse, T.,
2005.
-
- Physiology, role and neuropharmacology of
yawning. Laryngorhinootologie 84,345-351.
-
- Goldie, L., Green, J.M., 1961. Yawning and
epilepsy. J. Psychosom. Res. 5, 263-268.
-
- Goren, J.L., Friedman, J.H., 1998. Yawning
as an aura for an L-dopa-induced on in
Parkinson's disease. Neurology 50, 823.
-
- Greco, M., Baenninger, R., Govern, J., 1993.
On the context of yawning: when, where and why?
Psychol. Rec. 43, 175-183.
-
- Gschwend, J., 1977. Yawning in a case with
transsecting glioma of the pons. Fortschr.
Neurol. Psychiatr. Grenzgeb. 45, 652-655.
-
- Guggisberg, A.G., Mathis, J., Herrmann,
U.S., Hess, C.W., 2007. The functional
relationship between yawning and vigilance.
Behav. Brain Res. 179, 159-166.
-
- Guggisberg, A.G., Mathis, J., Hess, C.W.,
2010. Interplay between yawning and vigilance: a
review of the experimental evidence. In:
Walusinski, O. (Ed.), The Mystery of Yawning in
Physiology and Disease. Front. Neurol. Neurosci.
Karger, Basel.
-
- Guggisberg, A.G., Mathis, J., Schnider, A.,
Hess, C.W., 2011. Why do we yawn?: The
importance of evidence for specific yawn-induced
effects. Neurosci. Biobehav. Rev. 35,
1302-1304.
-
- Gutiérrez-Alvarez, A.M., 2007. Do
your patients suffer from excessive yawning?
Acta Psychiatr. Scand. 115 (January (1)),
80-81.
-
- Harada, K., 2006. Paroxetine-induced
excessive yawning. Psychiatry Clin. Neurosci.
60, 260.
-
- Hatakeyama, S., Kawai, Y., Ueyama, T.,
Senba, E., 1996. Nitric oxide synthase-
containing magnocellular neurons of the rat
hypothalamus synthesize oxytocin and vasopressin
and express Fos following stress stimuli. J.
Chem. Neuroanat. 11, 243-256.
-
- Hess, W.R., 1944. Das Schlafsyndrom als
Folge diencephaler Reizung. Helv. Physiol. Acta
2, 305-344.
-
- Heusner, A.P., 1946. Yawning and associated
phenomena. Physiol. Rev. 26, 156-168.
-
- Hipólide, D.C., Lobo, L.L., De
Medeiros, R., Neumann, B., Tufik, S., 1999.
Treatment with dexamethasone alters yawning
behavior induced by cholinergic but not
dopaminergic agonist. Physiol. Behav. 65,
829-832.
-
- Iacoboni, M., Koski, L.M., Brass, M.,
Bekkering, H., Woods, R.P., Dubeau, M.C.,
-
- Mazziotta, J.C., Rizzolatti, G., 2001.
Reafferent copies of imitated actions in the
right superior temporal cortex. Proc. Natl.
Acad. Sci. U. S. A. 98, 13995-13999.
-
- Jouvet, M., Bobillier, P., Pujol, J.-F.,
Renault, J., 1967. Suppression du sommeil et
diminution de la serotonine
cérébrale par lésion du
système du raphé chez le chat.
C.R. Acad. Sci. Paris 264, 360-363.
-
- Jurko, M.F., Andy, O.J., 1975. Post-lesion
yawning and thalamotomy site. Appl.
Neurophysiol. 38, 73-79.
-
- Karasawa, J., Kuriyama, Y., Kuro, M.,
Kikuchi, H., Sawada, T., Mitsugi, T., 1982.
Monitoring system of cerebral blood flow and
cerebral metabolism: part II. Relationship
between internal jugular O2 tention and cerebral
blood flow (author's transl). No To Shinkei 34,
239-245
-
- Karp, J.S., Hurtig, H.I., 1974. Locked-in
state with bilateral midbrain infarcts. Arch.
Neurol. 30, 176-178.
-
- Kita, I., Sato-Suzuki, I., Oguri, M., Arita,
H., 2000. Yawning responses induced by local
hypoxia in the paraventricular nucleus of the
rat. Behav. Brain Res. 117, 119-126.
-
- Kita, I., Yoshida, Y., Nishino, S., 2006. An
activation of parvocellular oxytocinergic
neurons in the paraventricular nucleus in
oxytocin-induced yawning and penile erection.
Neurosci. Res. 54, 269-275.
-
- Kosfeld, M., Heinrichs, M., Zak, P.J.,
Fischbacher, U., Fehr, E., 2005. Oxytocin
increases trust in humans. Nature 435,
673-676.
-
- Krasnianski, M., Gaul, C., Neudecker, S.,
Behrmann, C., Schlüter, A., 2003.
-
- Winterholler M. Yawning despite trismus in a
patient with locked-in syndrome caused by a
thrombosed megadolichobasilar artery. Clin.
Neurol. Neurosurg. 106, 44-46.
-
- Krestel, H., Weisstanner, C., Hess, C.W.,
Bassetti, C.L., Nirkko, A., Wiest, R., 2015.
Insular and caudate lesions release abnormal
yawning in stroke patients. Brain Struct. Funct.
220, 803-812.
-
- Kuba, R., Musilová, K.,
Brázdil, M., Rektor, I., 2010. Peri-ictal
yawning lateralizes the seizure onset zone to
the nondominant hemisphere in patients with
temporal lobe epilepsy. Epilepsy Behav. 19,
311-314.
-
- Kumar, N.N., Ferguson, J., Padley, J.R.,
Pilowsky, P.M., Goodchild, A.K., 2009.
Differential muscarinic receptor gene expression
levels in the ventral medulla of spontaneously
hypertensive and Wistar-Kyoto rats: role in
sympathetic baroreflex function. J. Hypertens.
27, 1001-1008.
-
- Kummer, W., Yamamoto, Y., 2002. Cellular
distribution of oxygen sensor candidates-
oxidases, cytochromes, K+-channels-in the
carotid body. Microsc. Res. Tech. 59,
234-242.
-
- López-Barneo, J.,
Ortega-Sáenz, P., Pardal, R., Pascual,
A., Piruat, J.I., 2008. Carotid body oxygen
sensing. Eur. Respir. J. 32, 1386-1398.
-
- Lal, S., Tesfaye, Y., Thavundayil, J.X.,
Skorzewewska, A., Schwartz, G., 2000. Effect of
time-of-day on yawning response to apomorphine
in normal subjects. Neuropsychobiology 41,
178-180.
-
- Laurell, K., Artto, V., Bendtsen, L., Hagen,
K., Häggström, J., Linde, M.,
Söderström, L., Tronvik, E., Wessman,
M., Zwart, J.A., Kallela, M., 2016. Premonitory
symptoms in migraine: a cross-sectional study in
2714 persons. Cephalalgia 36, 951-959.
-
- Laurent-Vannier, A., Fadda, G., Laigle, P.,
Dusser, A., Leroy-Malherbe, V., 1999. Foix-
Chavany-Marie syndrome in a child caused by a
head trauma. Rev. Neurol. (Paris) 155, 387-390
-
- LeWitt, P.A., Ondo, W.G., Van Lunen, B.,
Bottini, P.B., 2009. Open-label study assessment
of safety and adverse effects of subcutaneous
apomorphine injections in treating off episodes
in advanced Parkinson disease. Clin.
Neuropharmacol. 32, 89-93.
-
- Lehmann, H.E., 1979. Yawning: a homeostatic
reflex and its psychological significance. Bull.
Menninger Clinic 43, 123-136.
-
- Louboungou, M., Anderson, J.R., 1987.
Yawning scratching, and protruded lips:
differential conditionability of natural acts in
pigtail monkeys (Macaca nemestrina). Primates
28, 367-375.
-
- MacGregor, E.A., Wilkinson, M., Bancroft,
K., 1993. Domperidone plus paracetamol in the
treatment of migraine. Cephalalgia 13,
124-127.
-
- Manish, M., Veenu, S., 2007. Persistent
vegetative state. Neurology 68, 1635. Marcos,
B., Chuang, T.T., Gil-Bea, F.J., Ramirez, M.J.,
2008. Effects of 5-HT6 receptor antagonism and
cholinesterase inhibition in models of cognitive
impairment in the rat. Br. J. Pharmacol. 155,
434-440.
-
- Marder, E., Rehm, K.J., 2005. Development of
central pattern generating circuits. Curr. Opin.
Neurobiol. 15, 86-93.
-
- Mayer, C., 1921. Physiologisches und
Pathologisches über das Gähnen.
Ztschr. Biol. 73, 101.
-
- Medrano, V., Selles-Galiana, M.F.,
Fernández-Izquierdo, S., Mallada-Frechin,
J., Díaz-González, F.,
Piqueras-Rodríguez, L., 2005.
[Yawning and temporal lobe epilepsy]
[Article in Spanish]. Rev. Neurol. 41,
63-64.
-
- Meenakshisundaram, R.,
Thirumalaikolundusubramanian, P., Walusinski,
O., Sweni, S., 2010. Associated movements in
hemiplegic limbs during yawning. In: Walusinski,
O. (Ed.), The Mystery of Yawning in Physiology
and Disease. Front. Neurol. Neurosci. Karger,
Basel.
-
- Meister, I.G., Boroojerdi, B., Foltys, H.,
Sparing, R., Huber, W., Töpper, R., 2003.
Motor cortex hand area and speech: implications
for the development of language.
Neuropsychologia 41, 401-406.
-
- Melis, M.R., Argiolas, A., 2002. Reduction
of drug-induced yawning and penile erection and
of noncontact erections in male rats by the
activation of GABAA receptors in the
paraventricular nucleus: involvement of nitric
oxide. Eur. J. Neurosci. 15, 852-860.
-
- Melis, M.R., Argiolas, A., Gessa, G.L.,
1987. Apomorphine-induced penile erection and
yawning: site of action in brain. Brain Res.
415, 98-104.
-
- Melis, M.R., Stancampiano, R., Argiolas, A.,
1994a. Nitric oxide synthase inhibitors prevent
N-methyl-D-aspartic acid-induced penile erection
and yawning in male rats. Neurosci. Lett. 179,
9-12.
-
- Melis, M.R., Stancampiano, R., Argiolas, A.,
1994b. Penile erection and yawning induced by
paraventricular NMDA injection in male rats are
mediated by oxytocin. Pharmacol Biochem. Behav.
48, 203-207.
-
- Millán, P.A., Montes, M.I., Uribe,
C.S., Cabrera, D., Arboleda, A., 2008.
Biopercular syndrome: report of two cases and
literature review. Biomedica 28, 183-190
-
- Moore, J., 1942. Some psychological aspects
of yawning. J. Gen. Psychol. 27, 289-294.
-
- Muchnik, S., Finkielman, S., Semeniuk, G.,
de Aguirre, M.I., 2003. Yawning and temporal
lobe epilepsy. Medicina (B Aires) 63,
137-139.
-
- Nahab, F.B., Hattori, N., Saad, Z.S.,
Hallett, M., 2009. Contagious yawning and the
frontal lobe: an fMRI study. Hum. Brain Mapp.
30, 1744-1751.
-
- Nakamura-Palacios, E.M., Amodeo Bueno, O.F.,
Takahashi, R.N., Tufik, S., 2002. Acute or
chronic effects of cannabinoids on spontaneous
or pharmacologically induced yawning in rats.
Pharmacol. Biochem. Behav. 74, 205-212.
-
- Nash, J., 1942. Surgical Physiology. Thomas,
Springfield, pp. 92-115.
-
- Nordgren, R.E., Markesbery, W.R., Fukuda,
K., Reeves, A.G., 1971. Seven cases of
cerebromedullospinal disconnection: the
locked-in syndrome. Neurology 21,
1140-1148.
-
- Nowak, P., Labus, Kostrzewa, R.M., Brus, R.,
2006. DSP-4 prevents dopamine receptor priming
by quinpirole. Pharmacol. Biochem. Behav. 84,
3-7.
-
- O'Brien, C.P., 1996. Drug addiction and drug
abuse, In: Hardman, J.G., Limbird, L.E.,
Molinoff, P.B., Ruddon, R.W., Gilman, A.G.
(Eds.), The Goodman and Gilman's The
Pharmacological Basis of Therapeutics. 9th ed.
McGraw Hill, New York, pp. 557- 580.
-
- O'Sullivan, J.D., Lees, A.J., Hughes, A.J.,
1999. Yawning in Parkinson's disease. Neurology
52, 428.
-
- Pahwa, R., Koller, W.C., Trosch, R.M.,
Sherry, J.H., APO303 Study Investigators, 2007.
Subcutaneous apomorphine in patients with
advanced Parkinson's disease: a dose-escalation
study with randomized, double-blind,
placebo-controlled crossover evaluation of a
single dose. J. Neurol. Sci. 258, 137-143.
-
- Park, M.H., Kim, B.J., Koh, S.B., Park,
M.-K.-, Park, K.W., Lee, D.H., 2005. Lesional
location of lateral medullary infarction
presenting hiccups (singultus). J. Neurol.
Neurosurg. Psychiatry 76, 95-98.
-
- Penfield, W., Jasper, H., 1954. Epilepsy and
the Functional Anatomy of the Human Brain
Little. Brown & Co, Boston.
-
- Platek, S.M., Critton, S.R., Myers, T.E.,
Gallup, G.G., 2003. Contagious yawning: the role
of self-awareness and mental state attribution.
Brain Res. Cogn. Brain Res. 17, 223-227.
-
- Platek, S.M., Mohamed, F.B., Gallup Jr.,
G.G., 2005. Contagious yawning and the brain.
Brain Res. Cogn. Brain Res. 23, 448-452.
-
- Postert, T., Pöhlau, D., Meves, S.,
Nastos, I., Przuntek, H., 1996. Pathological
yawning as a symptom of multiple sclerosis. J.
Neurol. 243, 300-301.
-
- Prasad, H., 2008. Amelioration of
pathological yawning after tracheostomy in a
patient with locked-in syndrome: a
thermoregulatory approach. Eur. J. Neurol. 15,
e114 (author reply e115).
-
- Provine, R.R., Hamernik, H.B., 1986.
Yawning: effect of stimulus interest. Bull.
Psychon. Soc. 24, 437-438.
-
- Provine, R.R., Tate, B.C., Geldmacher, L.L.,
1987. Yawning: no effect of 3-5% CO2, 100% O2,
and exercise. Behav. Neural. Biol. 48,
382-393.
-
- Provine, R.R., 1986. Yawning as a
stereotyped action pattern and releasing
stimulus. Ethology 72, 109-122.
-
- Rimondini, R., Ferré, S.,
Giménez-Llort, L., Ogren, S.O., Fuxe, K.,
1998. Differential effects of selective
adenosine A1 and A2A receptor agonists on
dopamine receptor agonist-induced behavioural
responses in rats. Eur. J. Pharmacol. 347,
153-158.
-
- Rizzolatti, G., Craighero, L., 2004. The
mirror-neuron system. Annu. Rev. Neurosci. 27,
169-192.
-
- Russell, H., 1891. The Delsarte Series. US
Book company, New York.
-
- Rybak, I.A., Abdala, A.P., Markin, S.N.,
Paton, J.F., Smith, J.C., 2007. Spatial
organization and state-dependent mechanisms for
respiratory rhythm and pattern generation. Prog.
Brain Res. 165, 201-220.
-
- Sánchez, F., Alonso, J.R.,
Arévalo, R., Blanco, E., Aijón,
J., Vázquez, R., 1994.
- Coexistence of NADPH-diaphorase with
vasopressin and oxytocin in the hypothalamic
magnocellular neurosecretory nuclei of the rat.
Cell Tissue Res. 276, 31-34.
-
- Sandyk, R., 1987. Excessive yawning and
progressive supranuclear palsy. Int. J.
Neurosci. 34, 123-124.
-
- Sanna, F., Succu, S., Melis, M.R., Argiolas,
A., 2012a. Dopamine agonist-induced penile
erection and yawning: differential role of
D2-like receptor subtypes and correlation with
nitric oxide production in the paraventricular
nucleus of the hypothalamus of male rats. Behav.
Brain Res. 230, 355-364.
-
- Sanna, F., Argiolas, A., Melis, M.R., 2012b.
Oxytocin-induced yawning: sites of action in the
brain and interaction with
mesolimbic/mesocortical and incertohypothalamic
dopaminergic neurons in male rats. Horm. Behav.
62, 505- 514.
-
- Sato-Suzuki, I., Kita, I., Oguri, M., Arita,
H., 1998. Stereotyped yawning responses induced
by electrical and chemical stimulation of
paraventricular nucleus of the rat. J.
Neurophysiol. 80, 2765-2775.
-
- Sato-Suzuki, I., Kita, I., Seki, Y., Oguri,
M., Arita, H., 2002. Cortical arousal induced by
microinjection of orexins into the
paraventricular nucleus of the rat. Behav. Brain
Res. 128, 169-177.
-
- Sawchenko, P.E., Swanson, L.W., 1981.
Central noradrenergic pathways for the
integration of hypothalamic neuroendocrine and
autonomic responses. Science 214, 685-687.
-
- Sawchenko, P.E., Swanson, L.W., 1982.
Immunohistochemical identification of neurons in
the paraventricular nucleus of the hypothalamus
that project to the medulla or to the spinal
cord in the rat. J. Comp. Neurol. 205,
260-272.
-
- Schürmann, M., Hesse, M.D., Stephan,
K.E., Saarela, M., Zilles, K., Hari, R., Fink,
G.R., 2005. Yearning to yawn: the neural basis
of contagious yawning. Neuroimage 24,
1260-1264.
-
- Schroeder, J.E., Fischbach, P.S., Zheng, D.,
McCleskey, E.W., 1991. Activation of mu opioid
receptors inhibits transient high- and
low-threshold Ca2+ currents, but spares a
sustained current. Neuron 6, 13-20.
-
- Schulte, L.H., May, A., 2016. The migraine
generator revisited: continuous scanning of the
migraine cycle over 30 days and three
spontaneous attacks. Brain 139, 1987-1993.
-
- Seki, Y., Sato-Suzuki, I., Kita, I., Oguri,
M., Arita, H., 2002. Yawning/cortical activation
induced by microinjection of histamine into the
paraventricular nucleus of the rat. Behav. Brain
Res. 134, 75-82.
-
- Shamay-Tsoory, S.G., Tomer, R., Berger,
B.D., Aharon-Peretz, J., 2003. Characterization
of empathy deficits following prefrontal brain
damage: the role of the right ventromedial
prefrontal cortex. J. Cogn. Neurosci. 15,
324-337.
- Sherer, D.M., Smith, S.A., Abramowicz, J.S.,
1991. Fetal yawning in utero at 20 weeks
gestation. J. Ultrasound. Med. 10, 68.
-
- Shoup-Knox, M.L., Gallup, A.C., Gallup,
G.G., McNay, E.C., 2010. Yawning and stretching
predict brain temperature changes in rats:
support for the thermoregulatory hypothesis.
Front. Evol. Neurosci. 2, 108.
-
- Sicard, J.A., Paraf, J., 1921. Fou rire
syncopal et bâillements au cours de
l'encéphalite epidémique. Bull. et
mem. soc. med. hop. Paris 45, 232.
- Sicuteri, F., 1977. Dopamine, the second
putative protagonist in headache. Headache 17,
129-131.
-
- Singer, O.C., Humpich, M.C., Lanfermann, H.,
Neumann-Haefelin, T., 2007. Yawning in acute
anterior circulation stroke. J. Neurol.
Neurosurg. Psychiatry 78, 1253- 1254.
-
- Sleight, A.J., Boess, F.G., Bös, M.,
Levet-Trafit, B., Riemer, C., Bourson, A., 1998.
Characterization of Ro 04-6790 and Ro 63-0563:
potent and selective antagonists at human and
rat 5-HT6 receptors. Br. J. Pharmacol. 124,
556-562.
-
- Smith, J.C., Ellenberger, H.H., Ballanyi,
K., Richter, D.W., Feldman, J.L., 1991. Pre-
Bötzinger complex: a brainstem region that
may generate respiratory rhythm in mammals.
Science 254, 726-729.
-
- Succu, S., Spano, M.S., Melis, M.R.,
Argiolas, A., 1998. Different effects of omega-
conotoxin on penile erection, yawning and
paraventricular nitric oxide in male rats. Eur.
J. Pharmacol. 359, 19-26.
-
- Swanson, L.W., Sawchenko, P.E., 1983.
Hypothalamic integration: organization of the
paraventricular and supraoptic nuclei. Annu.
Rev. Neurosci. 6, 269-324.
-
- Swanson, L.W., Sawchenko, P.E.,
Bérod, A., Hartman, B.K., Helle, K.B.,
Vanorden, D.E., 1981. An immunohistochemical
study of the organization of catecholaminergic
cells and terminal fields in the paraventricular
and supraoptic nuclei of the hypothalamus. J.
Comp. Neurol. 196, 271-285.
-
- Thet, L.A., Clerch, L., Massaro, G.D.,
Massaro, D., 1979. Changes in sedimentation of
surfactant in ventilated excised rat lungs.
Physical alterations in surfactant associated
with the development and reversal of
atelectasis. J. Clin. Invest. 64, 600-608.
-
- Tizabi, Y., Copeland Jr, R.L., Brus, R.,
Kostrzewa, R.M., 1999. Nicotine blocks
quinpirole-induced behavior in rats: psychiatric
implications. Psychopharmacology (Berl.) 145,
433-441.
-
- Tuerke, K.J., Limebeer, C.L., Fletcher,
P.J., Parker, L.A., 2012. Double dissociation
between regulation of conditioned disgust and
taste avoidance by serotonin availability at the
5-HT(3) receptor in the posterior and anterior
insular cortex. J. Neurosci. 32,
13709-13717.
-
- Van Woerden, E.E., van Geijn, H.P., Caron,
F.J., van der Valk, A.W., Swartjes, J.M., Arts,
N.F., 1988. Fetal mouth movements during
behavioural states 1F and 2F. Eur. J. Obstet.
Gynecol. Reprod. Biol. 29, 97-105.
-
- Walusinski, O., Quoirin, E., Neau, J.P.,
2005. Parakinesia brachialis oscitans. Rev.
Neurol. (Paris) 161, 193-200 (Article in
French).
-
- Walusinski, O., 2006. Yawning: unsuspected
avenue for a better understanding of arousal and
interoception. Med. Hypotheses 67, 6-14.
-
- Walusinski, O., 2010. Fetal yawning. In:
Walusinski, O. (Ed.), The Mystery of Yawning in
Physiology and Disease. Front. Neurol. Neurosci.
Karger, Basel, pp. 32-41.
-
- Walusinski, O., 2014. How yawning switches
the default-mode network to the attentional
network by activating the cerebrospinal fluid
flow. Clin. Anat. 27, 201-209.
-
- Wei, J., Walton, E.A., Milici, A.,
Buccafusco, J.J., 1994. m1-m5 muscarinic
receptor distribution in rat CNS by RT-PCR and
HPLC. J. Neurochem. 63, 815-821.
-
- Weiss, N., Galanaud, D., Carpentier, A.,
Tezenas de Montcel, S., Naccache, L., Coriat,
P., Puybasset, L., 2008. A combined clinical and
MRI approach for outcome assessment of traumatic
head injured comatose patients. J. Neurol. 255,
217- 223.
-
- Wicks, P., 2007. Excessive yawning is common
in the bulbar-onset form of ALS. Acta Psychiatr.
Scand. 116 (76) author reply 76-77.
-
- Wilkinson, A., Sebanz, N., Mandli, I.,
Huber, L., 2011. No evidence of contagious
yawning in the red-footed tortoise. Curr. Zool.
57, 477-484.
-
- Williams, D.R., 2000. The yawning reflex: an
upper motor neuron sign in amyotrophic lateral
sclerosis. Neurology 55, 1592-1593.
-
- Wimalaratna, H.S., Capildeo, R., 1988. Is
yawning a brainstem phenomenon? Lancet 1,
300.
-
- Wimmer, A., 1924. Chronic Epidemic
Encephalitis. Wiliam Heinemann Medical Books,
London.
-
-
- Yankovsky, A.E., Andermann, F., Dubeau, F.,
2006. Post-ictal forceful yawning in a patient
with nondominant hemisphere epilepsy. Epileptic.
Disord. 8, 65-69.
-
- Zheng, H., Bidasee, K.R., Mayhan, W.G.,
Patel, K.P., 2007. Lack of central nitric oxide
triggers erectile dysfunction in diabetes. Am.
J. Physiol. Regul. Integr. Comp. Physiol. 292,
R1158-1164.
-
|





