- Abstract : Almost all the vertebrates
yawn, testifying the phylogenetic old origins of
this behavior. Correlatively speaking, yawning
shows an ontogenical precociousness since it
occurs as early as 12 weeks after conception and
remains relatively unchanged throughout life.
Thus, it is contended that these common
characteristics and their diencephalic origin
allow to model an approach from which emerges a
pivotal link between yawning and REM sleep.
Yawning and stretching reverse the muscular
atonia of the REM-sleep and reopen the collapsed
airways. Yawning appears as a powerful muscular
stretch, recruiting specific control systems
particularly the paraventricular nucleus of the
hypothalamus, the Locus Coeruleus and the
reticular activating system from which the vigor
of this ancestral vestige, surviving throughout
evolution with little variation, may increase
arousal.
-
- On the other hand, the
James-Lange theory proposes that afferent
feedback from muscles and viscera provides the
brain with a feeling that characterizes the
active motivational state and arousal. On this
basis and using selected supporting findings
from the literature and from data provided by
daily life, it is contended that yawning takes
part in interoceptiveness by its capacity to
increase arousal and self-awareness. Adaptative
behaviors depend on interactions among the
nervous system and the body by a continuous
feedback between them. The body's schema is a
main component of the self, and interoceptive
process is essential to awareness of the body
and arousal. Yawning contributes to bodily
consciousness as a behavior affiliating a
sensory motor act and his perception from which
pleasure is derived. Yawning can be seen as a
proprioceptive performance awareness which
inwardly provides a pre-reflective sense of
one's body and a reappraisal of the body schema.
The behavioral consequences of adopting specific
regulatory strategies and the neural systems
involved act upon attention and cognitive
changes.Thus, it is proposed that yawning is a
part of interoceptiveness by its capacity to
increase arousal and self-awareness.
-
Résumé : Il semble
qu'à peu près tous les
vertébrés bâillent, ce qui
témoigne de l'ancienneté
phylogenétique de ce comportement. En
corollaire, le bâillement se
caractérise par sa
précocité ontogénique
(récapitulation ontogenique ou loi
de von Baer) puisqu'il est détectable
chez le foetus dès 12 semaines
après la conception et qu'il perdure la
vie durant, sans changer d'aspect.
-
- Ces deux caractéristiques et son
origine diencéphalique permettent de
proposer une théorie montrant les liens
étroits unissant le bâillement et
le sommeil paradoxal. Bâillements et
pandiculations inversent l'hypotonie musculaire
et le collapsus des voies respiratoires
supérieures caractérisant le
sommeil paradoxal. Le bâillement
apparaît comme une puissante contraction
musculaire, activée par un système
neuronal comprenant le noyau
paraventriculaire de l'hypothalamus, le
locus coeruleus, et la réticulé
activatrice du tronc cérébral.
Toutes ces structures participent du
système du maintien et de la stimulation
de l'éveil, expliquant l'importance du
bâillement, vestige comportemental
ancestral.
-
- D'autre part, la théorie des
émotions de James-Lange propose que les
sensations provenant des muscles et des
viscères sont parmi les perceptions
nécessaires à l'activité
cérébrale tant pour l'éveil
que pour la conscience d'être. A partir de
ce concept et en collectant de multiples
données d'observations et de la
littérature, pourquoi ne pas concevoir le
bâillement comme un des
éléments constituant
l'intéroception par sa capacité
à stimuler l'éveil, la vigilance
et la conscience.
-
- De l'interaction permanente et
réciproque entre le cerveau et l'ensemble
du corps dépend l'élaboration de
comportements adaptés. Le schéma
corporel est un élément essentiel
du Soi. Le processus de l'intéroception
est essentiel à la vigilance et à
la conscience d'être. Le bâillement
participe aux mécanismes de la perception
conciente du corps comme comportement associant
une activité motrice sensoriellement
perçue à laquelle s'ajoute une
composante hédonique. Le bâillement
peut ainsi se concevoir comme un comportement
renforçant l'auto-perception du corps et
l'engramme du schéma corporel. D'autre
part, l'attention et la cognition
nécessitent des régulations
adaptatives comportementales spécifiques
(homéostasiques) sous-tendues par des
circuits neuronaux propres.
-
- L'agrégat de toutes ces
données permet de proposer que le
bâillement est un comportement adaptatif
visant à stimuler l'éveil et dont
la perception accroît la vigilance et la
conscience de soi.
-
«
I should like to work like the archeologist who
pieces together the fragments of a lovely thing
which are alone left to him. As he proceeds,
fragment by fragment, he is guided by the
conviction that these fragments are part of a
larger whole which, however, he does not yet
know »
- Hans
Spemann (1938).
-
- Introduction.
- Organisms exhibit cyclic variations in a
variety of essential functions, including the
sleep-wake cycle, feeding and reproduction,
secondary, for example, to the daily alternation
of darkness and light exerted by the rotation of
the earth. Yawning, one of the most
underappreciated of stereotyped behaviors,
appears to be associated with each behavioral
transition occurring at the beginning and the
end of these functions. Our purpose is to give a
new insight built on an evolutionary perspective
of the wake/sleep system, and in particular, to
argue that yawning shares links with REM sleep
and arousal. The properties of yawning, thus
revealed, help to give new explanations of its
mysterious functions and of its survival without
evolutionary variations postulating a particular
importance in terms of needs. One might assume
that yawning is a component of the interoceptive
processes, essential to awareness and arousal.
It is contended that yawning is a part of
interoception by its capacity to increase
arousal and self-awareness [2].
-
- Yawning: its cycles, its life.
- Ethologists agree that most vertebrates
yawn. Yawning is morphologically similar in
reptiles, birds, mammals and fishs. There are
three types of morphologically identical yawns
occurring in three distinct situations:
situations relative to circadian rest-activity
rhythms, situations relative to feeding,
situations relative to sexuality or social
interactions [3].
-
- Yawning is a stereotyped and often
repetitive motor act characterized by gaping of
the mouth accompanied by a long inspiration, a
brief acme followed by a short expiration. In
human, the expansion of the pharynx can
quadruple its diameter at rest diameter, while
the larynx opens up with maximal abduction of
the vocal cords. These characteristics cannot be
noticed in any other moment of life. Yawning is
not just a matter of opening one's mouth, but a
generalised stretching of muscles, those of the
respiratory tract (diaphragm, intercostal), the
face and the neck. It may be seen as a part of
the generalized stretch, named pandiculation,
with which it is generally associated
[4]. It is necessary to notice that the
function of stretching is actually not well
understood. This association of complex and
synergic movements occurs with an involuntary
occurrence and shares no criteria of a classical
reflex.
-
- When animals change between behaviors, they
are not merely responding in a passive way to
conditions of the environnement, like day-night
succession, for example. Rather, they are
following internally generated signals produced
by homeostasis procedures originating from the
hypothalamus (suprachiasmatic nucleus, SCN, and
paraventricular nucleus, PVN, of the
hypothalamus). This internal rhythm has the
ability to anticipate the transitions and
triggers behavioral and physiological changes in
accordance with those transitions. This
association has two advantages : predictability
and the possibility to detect the unexpected.
Yawning is a behavior which shares these
characteristics and appears to be associated
with transitions between periods of high and low
activity or arousal. A circadian pattern has
been found in spontaneous yawning. In normal,
unstressed humans daily peaks of yawning are
associated with transitions from sleeping to
waking and from waking to sleeping
[5,6].
-
- Yawning : neurophysiology.
- Until now, no specific cerebral structure
has been identified as a yawning centre. A good
number of clinical and pharmacological arguments
indicate that yawning involves the hypothalamus
(particularly the PVN),
the bulbus and pontic regions, with frontal
region connections in primates and to the
cervical medulla. The PVN is an integration
centre between the central and peripheral
autonomic nervous systems. It is involved in
numerous functions ranging from feeding,
metabolic balance, blood pressure and heart
rate, to sexual behaviour and yawning. In
particular, a group of oxytocinergic neurons
originating in this nucleus and projecting to
extra-hypothalamic brain areas (e.g.,
hippocampus, medulla oblongata and spinal cord)
controls yawning and penile erection. Activation
of these neurons by dopamine and its agonists,
excitatory amino acids (N-methyl-D-aspartic
acid) or oxytocin itself, or by electrical
stimulation leads to yawning, while their
inhibition by gamma-amino-butyric acid (GABA)
and its agonists or by opioid peptides and
opiate-like drugs inhibits yawning and sexual
response. The activation of these neurons is
secondary to the activation of nitric oxide
synthase, which produces nitric oxide. Nitric
oxide in turn causes, by a mechanism that is as
yet unidentified, the release of oxytocin in
extra-hypothalamic brain areas. Other compounds
modulate yawning by activating central
oxytocinergic neurons: sexual hormones,
serotonin, hypocretine and endogenous peptides
(adrenocorticotropin-melanocyte-stimulating
hormone). Oxytocin activates cholinergic
neurotransmission in the hippocampus and the
reticular formation of the brainstem
[7,8]. Acetylcholine induces yawning via
the muscarinic receptors of effectors from which
the respiratory neurons in the medulla, the
motor nuclei of the Vth,VIIth, IXth, Xth, and
XIIth cranial nerves, the phrenic nerves (C1-C4)
and the motor supply to the intercostal
muscles.
-
- Yawning: ontogenesis.
- The facial bone structure and the brain
become distinct starting from a common embryonic
structure, the ectoblast. The cephalic pole
comprises an original embryological
encephalo-facial and encephalo-cervical
segmentation with a strict topographical
correspondence: the naso-frontal and
premaxillary structures are joined to the
anterior brain; the maxillo-mandibular and
anterior cervical structures are joined to the
brainstem and its nerves. At the beginning of
the third month, the embryo becomes a fetus with
the occurrence of the first oral and pharyngal
motor sequences under the control of the
neurological development of the brainstem. The
development of the suction-deglutition and
yawning activity, sharing the same embryological
origin, shows the importance of the brainstem in
the neurophysiological development of the
oropharyngeal activity coordinated with the
respiratory, cardiac and digestive regulations
which have the same neuroanatomical localization
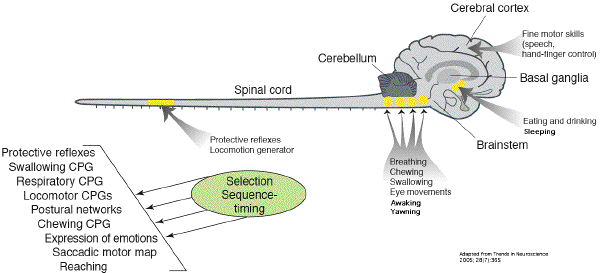
[9,10]. These circuits that generate
organized and repetitive motor patterns, such as
those underlying feeding, locomotion and
respiration belong to the Central Pattern
Generators in the medulla (CPG) which are
genetically determined, subserving innate motor
behaviours essential for survival [11].
Although in higher primates CPG are partialy
under neocortical control, reflexive control
systems involving CPG contribute to swallowing,
breathing and cough [12] which are all
dependent on pharyngo-laryngeal muscles control
[13]. Thus, it is argued that yawning
takes part of this CPG for his motor aspect.
Afferent somatosensory feedbacks, for both
temporal coordination and intensity, provide
simultaneous visceral sensation and autonomic
response (PVN) by which yawning take part of the
arousal homeostasis [14].
- Yawning and stretching have the original
traits of related phylogenetic old origins and,
as correlates, ontogenetic precociousness.
Rhythmic motor patterns and movements are seen
embryonically, before they are needed for
behavior from which it is suggested that
activity in immature networks is important for
circuit formation and transmitter specification
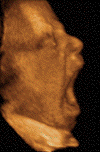
[11]. In the human embryo, yawning
occurs as early as 12 weeks after conception and
remains relatively unchanged throughout life.
Its survival without evolutionary variations
postulates a particular importance in terms of
developmental needs. The strong muscular
contraction that signifies a yawn is
metabolically expensive. If we accord with the
terms of Darwin's evolutionary propositions, the
costs of brain activity must be outweighed by
the advantages gained in terms of developmental
fitness. Thus, a structural hypothesis suggests
activation in the synthesis of neurotrophins,
which lead to a cascade of both new synapse
formation or recruitment, and activation through
the diencephalon, brainstem, and spinal cord.
The phenomenon of activity-dependent development
has been clearly shown to be one mechanism by
which early sensory or motor experience can
affect the course of neural development
[15]. The ability to initiate motor
behavior generated centrally and linked to
arousal and respiratory function is a property
of the brainstem reticular formation, which has
been remarkably conserved during the phylogeny
of vertebrates including agnathans, fishes,
amphibians, reptiles, and birds [16,17].
Therefore, conservative developmental mechanisms
orchestrating the organogenesis of the brainstem
in all vertebrates are probably crucial for
arousal and breathing.
-
- As an example, it is possible to compare
Ondine's syndrom, congenital or acquired (Chiari
malformation) with the locked-in syndrome. It
allows to distinguish brainstem from
supramedullary regulatory mechanisms in humans.
The former comprises loss of autonomic
respiratory control, requires volitional
breathing for survival, and points out the loss
of any yawn. The latter entails loss of
corticospinal or corticobulbar tracts required
for volitional breathing, preserves autonomic
respiratory control and characterizes
automatic-voluntary dissociation with tenacious
yawns [18]. Thus, yawning provides
evidence for the emergences of stereotyped
inborn fixed action patterns which may reappear
as pathological states: epilepsy, stroke
[19,20,21].
-
- Sleep, arousal and yawning.
- The phylogenetic
appearance of sleep proposes that the nocturnal
resting in poikilotherms most probably manifests
in mammals as a form of rapid eye movement (REM)
sleep or paradoxical sleep, which is
characterized by peripheral muscular atonia
originating in the dorsal part of the brainstem,
rostral to the pons [22]. Based on
numerous studies of fetuses and infants in a
variety of mammalian species, it is widely
believed that the earliest form of sleep is
properly characterized as active sleep, that is
an immature form of REM sleep and preponderant
at birth. Accordingly, it is thought that quiet
sleep, an immature form of slow-wave sleep
(SWS), emerges as REM sleep's predominance
diminishes during ontogeny
[23,24,25].
-
- In the early intra-uterine life, a diffuse
collection of phasic and cyclic motor events
occur that gradually coalesce. For the fetus,
sleep and wakefulness are reliably
characterized, respectively, by periods of
myoclonic twitching expressed against a
background of muscle atonia and high-amplitude
behaviors (e.g., locomotion or
stretching-yawning) expressed against a
background of high muscle tone. Movements of the
limbs, such as stretching, yawning, and kicking,
are typically considered to indicate periods of
wakefulness [26]. Periods of twitching
are almost always followed by the abrupt onset
of high-amplitude awake behaviors, thus
completing the cycle. Although myoclonic
twitching during active sleep in infants is more
prevalent and more intense than that seen during
REM sleep in adults, its similarities to the
adult behavior and its linkage to periods of
atonia suggest developmental continuity between
the infant and adult sleep states. The
maturation of the central nervous system, based
on myelinization, starts in the spinal cord and
then proceeds to the brainstem and forebrain.
Thus, paradoxical sleep mechanisms located in
the brainstem are the first to mature and the
only ones to function. Then, the slow-wave sleep
and waking structures become mature
[27,28,29]. Namely, the widespread
control of neuronal activity exerted by specific
REM sleep processes helps to direct brain
maturation through activity-dependent
developmental mechanisms. It may be inferred
that REM sleep (and possibly yawning) directs
the course of brain maturation in early life
through the control of neural activity
[11]. Behavioral pattern continuity from
prenatal to postnatal life shows a strict
parallelism between the ontogeny of REM sleep
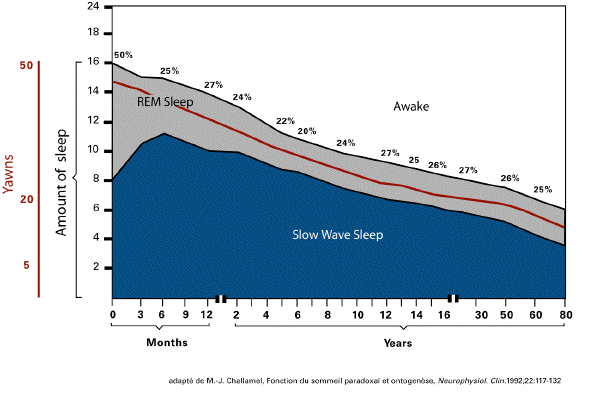
and yawning. Basically, REM sleep in the human
declines from 50% of total sleep time (8 h) and
a frequency of 30/50 yawns per day, in the
newborn, to 15% of total sleep time (1 h) and
less than 20 yawns per day, in the adult. This
decrease takes place mainly between birth and
the end of puberty. The emergence of distinct
states is followed by dramatic changes in the
amounts, duration, and cyclicity. An ultradian
rhythm may be graded; in a period from 50 to 60
minutes appears an alternation of moment
characterized by motor activity and by rest, as
in newborns. Each period of rest switches over a
period of activity by a yawn. Thus a periodicity
of one or two yawns per hour can be noticed.
Yawning appears 2 weeks before any discernible
sleep-wake states, and its expression gradually
becomes linked. No changes in the incidence of
yawns between 20 and 36 weeks of gestational age
have been observed by Roodenburg [30] in
the fetus. In preterm and full-term infants,
yawns are frequently observed on the first days
of life [31].
-
- Thus, the REM sleep and the yawning-stretch
syndrome, are two opposite muscles tones,
ontologically linked, and may be seen as
ancestral vestiges surviving throughout
evolution with little variation. Decades ago,
McLean postulated that these behavioral
routines, similar across vertebrates, are
evolutionarily conserved and mediated by the
similarly conserved basal ganglia and related
brain systems. Yawning is an example which
validates McLean's postulates testifying that
human behavioral medicine can profit from a
broad comparative approach [32].
 - Yawning and awaking.
- Sleep is a reversible behavioral state of
perceptual disengagment from and
unresponsiviness to the environnement but also
the inner state. The sensory inputs and motor
outputs are simultaneously blocked when the
brain is activated during REM sleep, putting it
off-line [33]. The preferred time to
wake up from sleep is phase related to circadian
rhythms. It is suggested that the homeostatic
component of sleep regulation dominates in the
first half of sleep, while the consistency in
the second half of sleep mainly depends on
circadian components. Awakenings show a
characteristic distribution with a maximum
immediately following REM sleep. This time
preferentially coincided with the rising slope
of the circadian rhythm of deep body temperature
[34,35]. Campbell [36] found
that sleep termination did not follow a
completed REM sleep episode but rather
interrupted REM sleep. He proposes "REM sleep as
a state with high neural activity which provides
optimal physiological conditions for the
transition from sleep to waking" [36].
The transition from sleep to waking implies a
physiological process which leads to a new
behavioral state. Awakening essentially
constitutes cortical arousal and is revealed by
electroencephalographic desynchronization and a
general increase of electrical excitability both
in sensory and motor systems [37]. The
activating system [38] is constituted by
neurons located in midbrain reticular formation
(the reticular activating system, RAS)
projecting to the thalamus and to the cortex
[39]. An intrinsic function of the RAS
is its participation in responses such that
alerting stimuli simultaneously activate
thalamocortical systems, as well as postural and
locomotor systems, in order to enable an
appropriate response (fight versus flight).
Neurons are, in the majority, noradrenergic and
particularly concentrated in small nuclei like
the locus coeruleus, having widespread
projections to forebrain areas and to virtually
all brain regions. locus coeruleus activity
varies first and foremost with the state of
vigilance, as first reported in 1969 by Jouvet
[40] and has a role in regulating
different types of cognitive abilities during
alertness. locus coeruleus neurons show low
activity during low vigilance behavioral states
such as grooming, but respond phasically to
stimuli in all sensory modalities when they are
novel and salient. The system contributes to the
initiation and maintenance of behavioral
activity necessary for the collection of sensory
information and stays as a critical component of
the central neural architecture supporting
interaction with and navigation through the
world [41].
-
- If REM sleep may facilitate for the brain a
smooth transition to wakefulness, it must be
noticed that REM sleep is characterized by a
peripheral muscular hypotonia (potent tonic
suppression) which may immediately switch to a
reversible state of basal muscle tone. It is
suggested that the trigemino-cervical-spinal
projections on the locus coeruleus, which convey
afferent stimulations, resulting from the
yawning-stretch syndrome, would favor behavioral
adjustment, through an enhancement of
'bottom-up' information processing. This signal
would have a general reset function. His
activation is tightly related to stimulus and
induces cognitive shifts by promoting reset of
functional networks [42]. Each motor
pattern is controlled by a specific functional
network, defined as a dynamic assembly of
neurons establishing specific spatiotemporal
interactions. The powerful muscular contraction
involved in the yawning-stretch syndrome
triggers an abrupt dissolution of the
preexisting functional network controlling the
REM sleep motor pattern and facilitates the
emergence of a functional network controlling
the awaking motor pattern. Reconfiguration of
networks is thus snappily achieved and their
reorganization promotes rapid behavioral
adaptation [43].
-
- To recapitulate, at becoming awake, yawning
and stretching reverse the muscular atonia which
characterize REM sleep. The wide inspiration
triggered by the yawn, which can be seen as a
form of sigh, improves lung compliance by
ensuring re-inflation of collapsed airways and
alveoli.
-
- Drowsiness and fatigue may be linked to the
dysruption of neural networks involved in tonic
attention, such as the reticular activating
system and related structures involved in the
subcortical attentional network. In the course
of the day, muscle tone tends to diminish as
drowsiness approaches and the upper airway would
tend to be drawn inwards. The stretching of
skeletal muscles would tend, on one hand, to
overcome the reduction of muscle tone in the
"antigravity" muscles and, on the other hand, to
restore normal airway resistance
[44].
-
- Schema
agrandi
-
- How yawning is triggered ?
- Awareness and more precisely arousal, are
essential components of total consciousness.
They require the ability to integrate sensory
informations from external environment, from
internal bodily states and modulation by
emotions and memory.
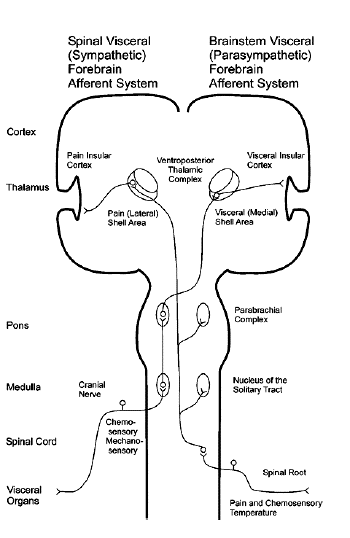
- The trigeminal nerve, the facial nerve, the
glossopharyngeal nerve, the vagus nerve and the
C1-C4 spinal nerves provide sensory information
and terminate topographically in the nucleus of
the solitary tract (NTS). NTS is involved in
central integration for the regulation of
arousal, sexuality and feeding. The major
outputs from the NTS is the parabrachial nucleus
which in turn provides extensive projections to
a wide range of sites in the brainstem,
hypothalamus, basal forebrain and thalamus. The
NTS and the parabrachial nucleus project to the
cerebral cortex, especially the insular visceral
sensory field, the amygdala, the sensory and
laterofrontal cortex. A part of the NTS's
neurons projects directly to the locus
coeruleus, the hypothalamus, mideline thalamic
nuclei, each of which has direct and diffuse
cortical projections. Sensory afferents from the
musculoskeletal joints converge via the
spinothalamic and the spinoreticular tracts
which passes through the brainstem and have two
divisions. The medial pathway, coming from
diaphragm, projects to the thalamic formation
and caudal raphe nuclei and then towards
cortical sensory regions. Many afferents end in
the parabrachial subnucleus, which provides a
diffuse input to the intralaminar thalamic
nuclei and thus is involved in arousal response
to musculoskeletal and visceral stimuli. A key
feature of this ascending pathway is that it
provides collaterals that converge with the
cranial nerve sensory pathways at virtually
every level. Some of the afferents may be
responsible for autonomic reflex responses to
visceral stimuli, and it is argued to yawning.
To keep in account, the thalamic nucleus and the
PVN belong to a neural loop circuitry sending
and receiving histaminergic projections from the
tuberomammillary nucleus, and noradrenergic
projections from the locus coeruleus. The basal
ganglia, as a rule, are highly interconnected
with the peduculonpontine tegmental nucleus
(PPN). PPN shows motor function by controlling
postural muscle tone and plays a role for the
regulation of the sleep-wake cycle and is a
limbic-motor interface for reward predictions
[45,46].
-
- Taking together, these charateristics
suggest that the visceral and musculoskeletal
sensory pathways are connected to the same
subcortical structures that provide arousal and
attention mechanisms [47]. Under this
perspective, yawning triggers the stimulation of
the locus coeruleus beyond musculoskeletal and
visceral sensory inputs.
-
- For example, the control of muscle tone of
the neck (trapezius) and of the masseters is one
of the elements contributing to the triggering
of our awakening [48]. The modification
of this tone would be one of the triggering
events of yawning. During the powerful
contraction caused by yawning, the spindles of
the masticatory muscles (masseters, temporal,
pterygoids), which have receptors that respond
to stretching, send stimuli via afferent nerve
of the Ia category, which are located in the
mesencephalic root of the trigeminal nerve
(ascending visceral parasympathic pathway). With
the motor neurons of the same muscles these
nerves form a monosynaptic link. This is the
basis of the masseteric reflex. These nerves
have projections on the RAS and the locus
coeruleus which are anatomically close to the
nucleus of the trigeminal nerve. Through the
massive contraction of the masseteric muscles,
yawning stimulates those structures responsible
for cortical activation. The fact that the
amplitude of the masseteric reflex varies in
parallel with the level of vigilance constitutes
another argument [49].
-
- What is interoception ?
- School children are still routinely taught
that there are five senses (sight, hearing,
touch, smell, taste, a classification first
devised by Aristotle). But it may be argued that
there are at least six different senses in
humans. The five senses belong to what is called
exteroception, the perception of stimuli which
come from an external source. Nociception, the
perception of pain, is a distinct phenomenon
that intertwined with all other senses,
including touch. In addition, some animals have
senses that humans do not, including the
following: electroreception, magnetoreception,
echolocation.
- By contrast, the sixth sense is the
interoception, the sensory perceptual process
for events occurring inside the body. It is the
perception of body awareness and frequently not
aware. The term "interoception" was introduced
in 1905 by Sherrington [50]. It includes
proprioceptive sensations and labyrinthine
functions but refers also much more broadly to
all bodily sensations, most frequently at the
border of consciousness [51].
-
- Yawning : the inside story.
- There are reciprocal connections between
insula and thalamus, hypothalamus, RAS, the
locus coeruleus. Yawning engages any of these
structures related to the representation and/or
regulation of organism state, for example, the
brainstem, the hypothalamus and the insula.
These regions share a major feature in that they
are all direct and indirect recipients of
signals from the internal milieu, visceral and
musculoskeletal frame. In addition, some
brainstem nuclei, the hypothalamus, and
subsectors of the insula and cingulate, also
generate regulatory signals necessary to
maintain homeostasis. The results underscore the
close anatomical and physiological connection
between yawning and homeostasis, and between
yawning and mapping of the ongoing state of the
organism. The neural patterns depicted in all of
these structures constitute multidimensional
maps of the organism's internal state and they
form the basis for an aspect of the feeling
state. Some of these maps, such as those in
brainstem and hypothalamus, are coarse. The maps
in insula and cingulate regions that receive
regulatory signals from brainstem and
hypothalamus in addition to direct sensory
signals from the organism, are more refined, and
their information is accessible to
consciousness, thus providing integrated
perceptual maps of the organism state
[52].
-
- After a yawn, humans experience an unfolding
feeling of well-being. Physical movement
(somatic motor system) and respiratory activity
are coordinated by interactions involving
brainstem mechanisms and structures such the
NTS, the PVN and the RAS. Visceral-somatic
sensations are functionnally and anatomically
linked. Subjectively experienced feelings as
well as emotions might be bases on higher-order
re-representations of homeostatic afferent
sensory activity in human forebrain. Direct
ascending projections from these sites activate
insular cortex by way of the basal
(parasympathetic) and posterior (sympathetic)
parts of the ventromedial nucleus of the
thalamus. These modality-specific,
topographically organized projection pathways
are phylogenetically distinct to primates and
are well-developed only in humans. These
pathways progressively activate higher-order
homeostatic afferent re-representations in more
anterior portions of the insula. The anterior
insula (particularly right, non dominant) is
activated predominantly by homeostatic
afferents. Indeed, the insular cortex is
involved in higher somatic integration, in
relation to both somatic, autonomic and limbic
systems [53]. The ventral anterior
insula is most important for core affect, a term
that describes broadly-tuned motivational states
with associated subjective feelings
[54].
-
- From the neurochemical point of view,
serotonin is known to modulate the regulation of
the sleep/wake cycle. Serotonergic (5HT) neurons
are found in the hypothalamus and the raphe
nuclei. These neurons innervate many different
regions of the brain and spinal cord, and play
also, important modulatory roles in regulating
locomotor coordination, neuro-endocrine systems,
motivation and reward, emotional balance, mood,
attention, and social behavior [55]. It
is argued that this serotonergic system is
involved in the well being induced by
yawning-reward. Thus, psychotropic drugs, such
the selective serotonin reuptake inhibitors, has
given a rich iatrogenic pathology, triggering
yawns salvos.
-
- Based on these numerous lines of evidence,
it is proposed that yawning associated with
arousal indirectly activates insula, anterior
cingulate cortex and somato-sensory cortex.
Subjective ratings of feeling from yawning are
correlated with homeostatic afferent activity,
including pleasant feeling. The capacity of
extract informations from this well-being, stays
as a substrate for subjective awareness of being
aware, consistent with the James-Lange theory of
emotion [56,57] and Damasio's somatic
marker hypothesis of consciousness
[58,59]. Yawning appears "one body
perspective experiment" and gives the
opportunity to enhance responses of the bodily
frame to higher cognitive level (brain's
representation of the body). Yawning plays a
multi-level role in that it not only stimulates
arousal but also regulates the level of
alertness and the ability to perform adaptively
during the waking state by resetting the
representation of body configuration
[60].
-
- Tentative conclusions.
- The development of adaptive behavior
includes not just an interaction between the
brain and the environment external to the
organism, but also the ongoing involvement of
the body in this process in both motor and
sensory aspects. Damasio postulates that
consciousness arose as a consequence of sensory
processes and argues that visceral sensations
contribute to the development of consciousness.
He attributes importance to interoceptive
processes as a general factor in ongoing
organismic functioning. Bodily input provides
stability, contributing to the sense of the self
as consistent and persistent over time. The
body's schema is a main component of the self
and interoceptive processes that is essential to
awareness of the body. Total muscle relaxation
appears to lead to loss of conscious imagery and
phantom limb phenomenon depends on the
persistence of sensory feedback produced by
residual muscular activity. Thus, it may be
argued that the sensory and motor systems are
one system and cognitive functions apparently
are related to motor processes. A sensory
experience would imply a motor response to issue
the consciousness of the self. Yawning
contributes to bodily consciousness as a
behavior affiliating a sensory motor act and his
perception from which pleasure is derived.
Yawning can be seen as a proprioceptive
performance awareness which inwardly provides a
pre-reflective sense of one's body and a
reappraisal of the body schema. It displays
three levels: embodiment (constrained and
enabled by motoric possibilities), communication
(making public an arousal state), cognition
(feeling well and rewarding) and remaps the link
unifying body and mind. Yawning connects
consciousness as well as unconscious (or
subconscious) interoception to higher mental
functions [61,62,63].
-
- Acknowledgements.
- I thank Tsung O. Cheng, M.D. (Professor of
Medicine George Washington University Medical
Center, 2150 Pennsylvania Avenue, N.W.,
Washington, D.C. 20037) for kindly reading and
correcting this text.
-
 - References.
- 1. Spemann H (1869-1941). Embryonic
development and induction. Yale Univ Press. New
Haven. 1938. 401p.
-
- 2. Cameron OG. Visceral sensory
neuroscience: interoception. Oxford University
Press. New York. 2002;357p.
-
- 3. Walusinski
O, Deputte B. The phylogeny, ethology and
nosogeny of yawning. Rev Neurol (Paris).
2004;160(11): 1011-1021.
-
- 4. Baenninger
R. On yawning and its functions. Psychonomic
Bul Rev. 1997;4(2):198-207.
-
- 5. Baenninger
R., Binkley S., et al. Field observations of
yawning and activity in humans. Physiol Behav.
1996; 59:421-425.
-
- 6.Provine
RR Yawning. American Scientist. 2005;93(6):
532-539.
-
- 7. Argiolas
A, Melis MR. The neuropharmacology of
yawning. Eur J Pharmacol. 1998;343(1):1-16.
-
- 8. Sato-Suzuki
I, Kita I, Oguri M, Arita H. Stereotyped
yawning responses induced by electrical and
chemical stimulation of paraventricular nucleus
of the rat. J Neurophysiol.
1998;80(5):2765-2775.
-
- 9. Borday C, Wrobel L, Fortin G, Champagnat
J, Thaeron-Antono C, Thoby-Brisson M.
Developmental gene control of brainstem
function: views from the embryo. Prog Biophys
Mol Biol. 2004;84(2-3):89-106.
-
- 10. Rogers B, Arvedson J. Assessment of
infant oral sensorimotor and swallowing
function. Ment Retard Dev Disabil Res Rev.
2005;11(1):74-82.
-
- 11. Marder E, Rehm KJ. Development of
central pattern generating circuits. Curr Opin
Neurobiol. 2005;15(1):86-93.
-
- 12. Straus C, Vasilakos K, Wilson RJ, Oshima
T, Zelter M, Derenne JP, Similowski T, Whitelaw
WA. A phylogenetic hypothesis for the origin of
hiccough. Bioessays. 2003;25(2):182-188.
-
- 13. Ludlow CL. Central nervous system
control of the laryngeal muscles in humans.
Respir Physiol Neurobiol.
2005;147(2-3):205-222.
-
- 14. Saper CB, Cano G, Scammell TE.
Homeostatic, circadian, and emotional regulation
of sleep. J Comp Neurol. 2005;493(1):92-98.
-
- 15. Lagercrantz H, Ringstedt T. Organization
of the neuronal circuits in the central nervous
system during development. Acta Paediatr.
2001;90(7):707-715.
-
- 16. Jacob J, Guthrie S. Facial visceral
motor neurons display specific rhombomere origin
and axon pathfinding behavior in the chick. J
Neurosci. 2000;20(20):7664-7671.
-
- 17. Chatonnet F, Thoby-Brisson M, Abadie V,
Dominguez del Toro E, Champagnat J, Fortin G.
Early development of respiratory rhythm
generation in mouse and chick. Respir Physiol
Neurobiol. 2002;131(1-2):5-13.
-
- 18. Ochoa-Sepulveda
JJ, Ochoa-Amor JJ. Ondine's curse during
pregnancy. J Neurol Neurosurg Psychiatr. 2005;
76; 294.
-
- 19. Meletti S, Cantalupo G,
Stanzani-Maserati M, Rubboli G, Tassinari AC.
The expression of interictal, preictal, and
postictal facial-wiping behavior
-
- 20. Tassinari CA, Rubboli G, Gardella E,
Cantalupo G, Calandra-Buonaura G, Vedovello M,
Alessandria M, Gandini G, Cinotti S, Zamponi N,
Meletti S. Central pattern generators for a
common semiology in fronto-limbic seizures and
in parasomnias. A neuroethologic approach.
Neurol Sci. 2005;26 Suppl 3:s225-232.
-
- 21.Walusinski O,
Quoirin E, Neau JP. Parakinesia brachialis
oscitans. Rev Neurol (Paris).
2005;161(2):193-200.
-
- 22. Nicolau MC, Akaarir M, Gamundi A,
Gonzalez J, Rial RV. Why we sleep: the
evolutionary pathway to the mammalian sleep.
Prog Neurobiol. 2000;62(4):379-406.
-
- 23. Blumberg MS, Luca DE. A developmental
and component analysis of active sleep. Develop
Psychobiol. 1996;29(1):1-22.
-
- 24. Valatx JL. The ontogeny and physiology
confirms the dual nature of sleep states. Arch
Ital Biol. 2004;142(4):569-580.
-
- 25. Siegel
JM. Sleep phylogeny : clues to the evolution
and function of sleep. In Luppi PH ed. Sleep :
circuits and functions. CRC Press. Boca Raton.
2005. 163-176.
-
- 26. Walusinski
O, Kurjak A, Andonotopo W, Azumendi G. Fetal
yawning assessed by 3D and 4D sonography.
Utrasound Rev Obs Gyncecol.
2005;5(3):210-217.
-
- 27. Feng P. The developmental regulation of
wake/sleep system. In Neuroendocrine correlates
of sleep/wakefulness. Cardinali DR and
Pandi-Perumal SR Ed. Springer. New York. 2006.
3-18.
-
- 28. Kobayashi T, Good C, Mamiya K, Skinner
RD, Garcia-Rill E. Development of REM sleep
drive and clinicals implications. J Appl
Physiol. 2004;96:735-746.
-
- 29. Karlsson KA, Gall AJ, Mohns EJ, Seelke
AM, Blumberg MS. The neural substrates of infant
sleep in rats. PLoS Biol. 2005;3(5):e143.
-
- 30. Roodenburg PJ, Wladimiroff JW, van Es A,
Prechtl HF. Classification and quantitative
aspects of fetal movements during the second
half of normal pregnancy. Early Hum Develop.
1991;25:19-35.
-
- 31. Giganti F, Hayes MJ, Akilesh MR,
Salzarulo P. Yawning and behavioral states in
premature infants. Development Psychobiol.
2002;41(3):289-293.
-
- 32. A Tribute to Paul MacLean: The
neurobiological relevance of social behavior.
Physiol Behav. 2003;79(3):341-547.
-
- 33. Hobson JA, Pace-Schott EF. The cognitive
neuroscience of sleep: neuronal systems,
consciousness and learning. Nat Rev Neurosci.
2002;3(9):679-693.
-
- 34. Czeisler CA, Zimmerman JC, Ronda JM,
Moore-Ede MC, Weitzman ED. Timing of REM sleep
is coupled to the circadian rhythm of body
temperature in man. Sleep.
1980;2(3):329-346.
-
- 35. Pace-Schott EF, Hobson A. The
neurobiology of sleep: genetics, cellular
physiology and subcortical networks. Nat Rev
Neurosci. 2002;3(8):591-605.
-
- 36. Campbell SS. Spontaneous termination of
ad libitum sleep episodes with special reference
to REM sleep. Electroencephalogr Clin
Neurophysiol. 1985;60(3):237-242.
-
- 37. Skinner RD, Homma Y, Garcia-Rill E.
Arousal mechanisms related to posture and
locomotion. Prog Brain Res.
2004;143:283-298.
-
- 38. Moruzzi G, Magoun HW. Brain stem
reticular formation and activation of the EEG
(1949). J Neuropsychiatry Clin Neurosci.
1995;7(2):251-267.
-
- 39. Steriade M. Impact of network activities
on neuronal properties in corticothalamic
systems. J Neurophysiol. 2001;86(1):1-39.
-
- 40. Jouvet M. Biogenic amines and the states
of sleep. Science. 1969;163(862):32-41.
-
- 41. Aston-Jones G. Brain structures and
receptors involved in alertness. Sleep Med.
2005;6 Suppl 1:S3-7.
-
- 42. Serrao M, Rossi P, Parisi L, Perrotta A,
Bartolo M, Cardinali P, Amabile G, Pierelli F.
Trigemino-cervical-spinal reflexes in humans.
Clin Neurophysiol. 2003;114(9):1697-703.
-
- 43. Bouret S, Sara SJ. Network reset: a
simplified overarching theory of locus coeruleus
noradrenaline function. Trends Neurosci.
2005;28(11):574-582.
-
- 44. Ayappa
I., Rapaport D. The upper airway in sleep:
physiology of the pharynx. Sleep Med Rev.
2003;7(1):9-33.
-
- 45. Mena-Segovia J, Bolam JP, Magill PJ.
Peduculunpontine nucleus and basal ganglia:
distant relatives or part of the same family?
Trends Cogn Sci. 2004;27(10):585-588.
-
- 46. McHaffie JG, Stanford TR, Stein BE,
Coizet V, Redgrave P. Subcortical loops through
the basal ganglia. Trends Neurosci.
2005;28(8):401-407.
-
- 47. Stehberg J, Acuna-Goycolea C, Ceric F,
Torrealba F. The visceral sector of the thalamic
reticular nucleus in the rat. Neurosci.
2001;106(4):745-755.
-
- 48. Mori S, Iwakiri H, Homma Y, Yokoama T,
Matsuyama K. Neuroanatomical and
neurophysiological base of postural control. Adv
Neurol. 1995;67:289-303.
-
- 49. Aston-Jones G, Cohen JD. An integrative
theory of locus coeruleus-norepinephrine
function: adaptive gain and optimal performance.
Ann Rev Neurosci. 2005;28:403-450.
-
- 50. Sherrington
CS (1857-1952). The integrative action of
the nervous sytem. Yale Univ Press. New Haven.
1906. 412p.
-
- 51. Craig
AD. How do you feel ? Interoception; the
sense of the physiological condition of the
body. Nat Rev Neurosci. 2002;3(8):655-666.
-
- 52. Berlucchi G, Aglioti S. The body in the
brain: neural bases of corporeal awareness.
Trends Neurosci. 1997;20(12):560-564.
-
- 53. Flynn FG, Benson DF, Ardila A. Anatomy
of the insula functional and clinical
correlates. Aphasiology. 1999;13(1):55-78.
-
- 54. Saper
CB. The central autonomic nervous system:
conscious visceral perception and autonomic
pattern generation. Annu Rev Neurosci.
2002;25:433-469.
-
- 55. Bagdy G. Role of the hypothalamic
paraventricular nucleus in 5-HT1A, 5-HT2A and
5-HT2C receptor-mediated oxytocin, prolactin and
ACTH/corticosterone responses. Behav Brain Res.
1996;73(1-2):277-280.
-
- 56. James
W (1842-1910). What is an emotion? Mind.
1884;9:188-205.
-
- 57.Lange
KG (1834-1900). Om Sindsbevægelser et
psyko-fysiologisk Studie. Lund Ed.
Kjøbenhavn. Denmark. 1885, 91p.
-
- 58. Damasio AR. Somatic markers and the
guidance of behavior: theory and preliminary
testing. In Frontal lobe function and
dysfunction. Levin HS et al. Ed. Oxford
University Press. 1991. 217-229.
-
- 59. Damasio AR. The feeling of what happens:
body and emotion in the making of consciousness.
Heinemann Ed. Harcourt Brace. New York. 1999;
396p.
-
- 60. Chiel HJ, Beer RD. The brain has a body:
adaptative behavior emerges from interactions of
nervous system, body an environment. Trends
Neurosci. 1997;20(12):553-557.
-
- 61. Critchley HD, Mathias CJ, Dolan RJ.
Neuroanatomical basis for first and second-order
representations of bodily states. Nat Neurosci.
2001;4(2):207-211.
-
- 62. Morris JS. How do you feel ? Trends Cogn
Sci. 2002;6(8):317-319.
-
- 63. Critchley HD, Wiens S, Rotshein P,
Öhman A, Dolan RJ. Neural systems
supporting interoceptive awareness. Nat
Neurosci. 2004;7(2):189-195.
-
- Yawning
Surprising facts ans misleading myths about our
health Anahad O'Connor
-
- Sollier
Paul Le sens
musculaire Archives de Neurologie Tome XIV 1887.
81-101
|