Introduction : In the past decade,
the results of many studies have greatly
increased the understanding of the physiology
and pharmacology of cannabinoids in the central
and peripheral nervous systems. For example,
cannabinoid CBI and CB2 receptors and a subtype
CB1A, have been characterized, cloned and the
second messenger systems identified.
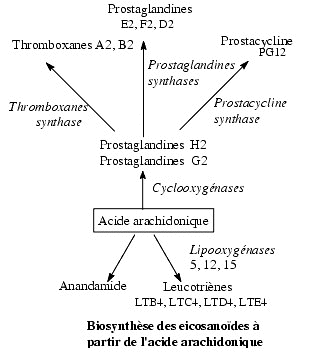
Anandamide, 2-arachidonyl glycerol,
homo--y-linolenylethanolamide,
7,10,13,16-docosatetraenyl-ethanolamide, mead
ethanolamide and palmitoylethanolamide have been
proposed as endogenous ligands for cannabinoid
receptors. The availability of cannabinoid
antagonists selective for the CB1 receptor,
SR141716A, and CB2 receptor, SR144528, has
greatly facilitated studies on the physiological
functions of cannabinoid systems. Additionally
other antagonists such as WIN 56,098,
6-bromopravodoline (WIN 54,461),
6-iodopravadoline (AM630), LY320135, have also
been synthesized and characterized.
Cannabinoids affect the actions and release
of many neurotransmitters, including
acetylcholine (ACh) and dopamine (DA). Recent
studies have demonstrated that cannabinoids act
at presynaptic CB1 receptors to inhibit ACh
release in ileal myenteric plexus-longitudinal
smooth muscle preparations, the hippocampus and
the medialprefrontal cortex. SR 141716A
antagonizes the inhibition of hippocampal ACh
release produced by cannabinoid agonists,
suggesting that the effects of cannabinoids on
learning and memory depend on a CB1
receptor-mediated inhibition of ACh release in
the hippocampus. Also, the combination of SR
141716A or delta9tetrahydrocannabinol
(delta9-THC) with scopolamine produced larger
disruptive effects. on a repeated-acquisition
procedure in squirrel monkeys than those
observed when either deltaTHC or scopolarnine
was administered alone, indicating that either a
CB1-receptor agonist or antagonist can alter the
disruptive effects of scopolamine on leaming in
squirrel monkeys.
In contrast to the inhibition of ACh release,
the stimulation of CB1 receptors produces an
activation of mesoprefrontal or mesolimbic
dopaminergic transmission. Because these
dopaminergic circuits are involved in the
reinforcing effects of most drugs of abuse, the
enhanced dopaminergic activity might underlie
the reinforcing and abuse properties of
marijuana. Additionally, the disruptive effects
of cannabinoids on cognitive processes might be
related to the activation of dopaminergic
transmission in the prefrontal cortex.
Adversely, the synthetic cannabinoid agonist,
HU 210, antagonized motor hyperactivity and
stereotypical behavior elicited by cocaine and a
DA receptor agonist, CQP 201 - 403. HU 210
also antagonized penile erection and
stretching-yawning elicited by dopaminergic
D2/D3 agonists, B-HT 920 and 7-OH-DPAT, in a
manner similar to that produced by a
dopaminergic D2 antagonist, ( - ) eticlopride.
Additionally, an intracerebroventricular
administration of an anandamide tranport
inhibitor N-(4-hydroxyphenyl)-arachidonamide
(AM404), which causes anandamide to accumulate
in the central nervous system, produced a mild
and slow-developing hypokinesia and reduced the
stimulation of motor behaviors elicited by the
selective D2 family receptor agonist quinpirole.
Therefore, it seems that cannabinoid agonists
can both increase and decrease dopaminergic
activity.
Yawning is a reflex, or stereotyped event
exhibited by all mammals and vertebrates. It
seems to be a brain stem arousal reflex with
both peripheral and central loops subserving
reversal of brain hypoxia or hypoxemia, probably
related to an effort to keep vigilance. Its
mechanisms and fonctional role are not entirely
known. It seems to be centrally linked with the
dopaminergic system in a D1 -D2 cooperation and
the cholinergic system as the effector pathway
for the dopaminergic -cholinergic linked neural
mechanism.
However, many other neurotransmitters and
neuropeptides, such as excitatory amino acids,
serotonin, gamaaminobutyric acid, noradrenaline,
nitric oxide, adrenocorticotropic hormone
related peptides, oxytocin and opioid peptides,
are also involved in the central control of
yawning.
The studies reviewed above demonstrate
that cannabinoids can alter transmission
mediated by both dopaminergic and cholinergic
pathways, both of which are involved in the
yawning response. Because cannabinoids are
known to alter responses mediated by these
neurotransmitter systems, the current study was
carried out to examine the effects of acute and
chronic treatments with cannabinoid agonists on
the spontaneous yawning response as well as that
produced by a cholinergic and dopaminergic
agonists in rats. [...]
Discussion :
The dose-dependent yawning induced by
cholinergic and dopaminergic agonists was
consistent with several studies. One could argue
that what we are callig as yawning might
actually be what is described as conditional
gaping in rats as a manifestation of the
vomiting response, relating our results to an
antiemetic effect of cannabinoids. However,
gaping behavior is characterized by a rapid
opening and closing of the mouth usually
accompanied by chin rubbing, reflecting an
aversive response or a rejection taste
reactivity response, in contrast to yawning, a
slower and wide opening of the mouth, sometimes
accompanied by stretching behavior, a pattern of
response observed in our study. Additionally,
the experimental observation of a conditional
rejection reactions in rats usually needs a
flavor, which will induce this response through
oral infusion, to be previously paired to an
emetic drug such as lithium chloride, a
procedure that was not used in our study.
Finally, there are very few reports in the
literature referring to cholinergic agonists
inducing gaping, or apomorphine inducing gaping
and this one in large doses, far beyond the dose
range employed in our study.
According to Yamada
and Furukawa (1980) and Ushijima
et al. (1984a), yawning induced by
pilocarpine is produced centrally, as shown by
the observation that yawning induced directly by
a cholinergic agonist such as pilocarpine or
indirectly by a cholinesterase inhibitor such as
physostigmine can be blocked by muscarinic
antagonists that penetrate the central nervous
system (e.g., scopolamine) but not by those
acting only peripherally (e.g.,
methylscopolamine).
Low doses of apomorphine act preferentially
at D2 presynaptic receptors to cause a reduction
in DA release. Considering the inhibitory
modulation of DA on ACh release, the reduced
release of DA could result in a greater release
of ACh, and thereby increase yawning. Centrally
acting muscarinic antagonists abolish yawning
induced by dopaminergic and cholinergic
agonists, whereas dopaminergic antagonists only
abolish apomorphineinduced yawning. These
observations strongly suggest that the yawning
induced by low doses of a dopaminergic agonist
is due to an increase in central cholinergic
transmission.
In the present study, acutely administered
delta8-THC or delta-9THC decreased yawning
induced by pilocarpine in a dosedependent manner
and completely blocked yawning induced by
apomorphine. It is likely that these actions
involve a cannabinoid modulation of central
dopaminergic and/or cholinergic systems, a
dopaminergic-cholinergic linked neural mechanism
or actions of the cannabinoids at another site
distal to that of pilocarpine.
The inhibitory effects of these cannabinoids
on pilocarpine-induced yawning cannot be readily
explained by the known ability of cannabinoid
agonists to inhibit ACh release or a competitive
interaction between the cannabinoid and
pilocarpine for occupancy of muscarinic
receptors. The cannabinoid agonists produced a
dose-dependent inhibition of the actions of
pilocarpine that could not be overcome by
increasing doses of pilocarpine. This form of
antagonisrn is analogous to that seen with the
inhibition of indirectly acting agonistsand
suggests that the cannabinoids are acting at a
site distal to the actions of pilocarpine.
Yawning induced by low doses of apomorphine
was much more sensitive to antagonism by both
d8-THC and d9-THC than that produced by
pilocarpine. Similar to what was observed for
pilocarpine, increasing doses of apomorphine did
not overcome the inhibition produced by d8-THC.
Apomorphine at 40µg/kg and pilocarpine at 2
mg/kg produced similar increases in yawning. The
lowest dose of d9-THC, 0.5 mg/kg, completely
abolished the response to apomorphine. In
contrast, this dose only produced about a 30%
decrease in the yawning produced by pilocarpine.
The inhibitory effects of the cannabinoid
agonists on the actions of apomorphine appear to
be due to an antagonism rather than an
enhancement of dopaminergic transmission.
Behavioral effects of apomorphine are
biphasic, low doses induce yawning and sedation,
probably by presynaptic D2 autoreceptors
activation, whereas higher doses induce
stereotypy and hyperactivity, probably by
postsynaptic D1 activation. These behaviors are
mutually exclusive in that yawning decreases
with increasing doses of apomorphine.
Several studies have shown that cannabinoid
agonists can either increase dopaminergic
activity or produce a dopaminergic
antagonistic-like effect. The antagonistic-like
actions of cannabinoid agonists might best
explain our results since the
apomorphine-induced yawning was the most
affected by cannabinoid agonists and no sign of
stereotypy or hyperactivity was observed in
animals treated with the combination of
cannabinoids with apomorphine.
This antagonistic-like action of cannabinoid
agonists may involve D2 dopamine receptor
mediation. Beltramo et al. (2000) recently
showed that both, anandamide transport inhibitor
AM404 and anandamide by itself counteract two
characteristic responses mediated by activation
of D2 family receptors, that is, yawning induced
by apomorphine in a dose equivalent to the
highest one employed in our study (80 µg/kg
sc) and quinpirole-induced stimulation of motor
behaviors. Because the stimulation of motor
behavior elicited by systemic administration of
the D2-like agonist quinpirole was increased by
SR 141716A, Giufftida et al. (1999) suggested
that the endocannabinoid system may modulate D2
dopamine-induced activation of psychomotor
activity acting as an inhibitory feedback
mechanism to this behavior.
Neurotransmitter or neuromodulator systems
other than cholinergic or dopaminergic also have
to be considered. According to Argiolas
and Melis (1998), activation of the
paraventricular nucleus of the hypothalamus by
DA, excitatory aminoacids and oxytocin
facilitates yawning by releasing oxytocin at
extrahypothalamic areas such as the hippocampus,
the pons and/or the medulla oblongata that play
a key role in the expression of this behavioral
event. The yawning induced by these
neurotransmitters or neuropeptides was only
antagonized by opioid peptides.
There were no changes in the ability of
pilocarpine or apomorphine to induce yawning at
24 h or 7 days after cessation of the chronic
administration of d8-THC. Previous studies on
yawning have shown that rodents treated
chronically with haloperidol, a dopaininergic
antagonist, exhibited a central hyposensitivity
to apomorphine and physostigmine, both of which
act via the release of ACh, but not with
pilocarpine, a directly acting agonist. On the
other hand, after chronic treatinent with
muscarinic antagonists such as atropine or
scopolamine there was a supersensitivity to
physostigmine and pilocarpine, but not to
apomorphine. Our results suggest that, unlike
their effects on other behaviors, chronic
treatment with a cannabinoid agonist does not
alter the sensitivity of systems modulating
yawning.
Recent studies have shown that the repeated
administration of cannabinoid agonists might or
might not change the acute effects of some
drugs. For example, Ferrari et al. (1999) found
that a short-term treatment (7 days) with a
cannabinoid agonist, HU 210, did not modify
cocaineinduced effects, although it increased
locomotor activity and stimulated escape
attempts produced by a D1/D2 agonist, CQP
201-403. A chronic administration of d9-THC (3
weeks) did not change the effects of amphetamine
or heroin in low-responder rats, but it
significantly increased the locomotor effects of
these drugs in high-responder rats.
Nevertheless, a salient finding of the
present study was that animals treated with
d8-THC for 30 days showed higher spontaneous
yawning 7 days after drug discontinuation
compared to animals treated with saline. The
latency for this effect of chronic cannabinoid
treatment is much less than that observed by
Lamarque et al. (2001). In their study, the
increased locomotor responses to heroin only
occurred in high-responder rats with a latency
of 41 days after cessation of treatinent for
heroin-treated rats. The basis for this
différence in latency of presumed
withdrawal signs is not known.
The lack of any changes in the sensitivity to
pilocarpine or apomorphine after cessation of
treatment suggests that the increased
spontaneous yawning observed in our study could
be due to changes in noncholinergic,
nondopaminergic neurotransmitter or
neuromodulator systems involved in yawning, such
as opioid peptides. Endogenous opioid peptides
seem to exert an inhibitory control on the
yawning response at the paraventricular
level. The repeated administration of
cannabinoid agonists produced a time-related
increase in proenkephalin gene expression and
mu-opioid receptor'activation of G-proteins in
the paraventricular nucleus, as well as in other
structures such as spinal cord, caudate-putamen,
nucleus accumbens, ventromedial nucleus of
hypothalamus, and pituitary. It is proposed
that the increase in spontaneous yawning
observed after cessation of cannabinoid
treatment might be related to the loss of an
increased tone at mu-opioid receptors.
Yawning is one of the nine signs in the
diagnostic criteria for opioid withdrawal
(DSM-IV-TR, 1994). The possible involvement of
an opioid system in spontaneous yawning
following cannabinoid withdrawal merits farther
investigation.
In summary, acute administration of d8-THC
or d9-THC significantly reduced yawning induced
by cholinergic or dopaminergic agonists.
Chronic exposure to the cannabinoid agonist did
not change yawning induced by cholinergic or
dopaminergic agonists 24 h or 7 days after drug
discontinuation. However, an increased
spontaneous yawning was observed 7 days after
cannabinoid withdrawal. This sign might provide
a good behavioral instrument for carrying out
studies on cannabinoid withdrawal and/or
dependence.