- Cannabinoid receptors, the target of the
marijuana constituent d9-tetrahydrocannabinol
(Pertwee, 1997), are densely expressed in basal
ganglia and cortex, regions of the CNS that are
critical forthe control of cognition,
motivation, and movement (Herkenhamet al., 1990;
Matsuda et al., 1993; Tsou et al., 1998). This
distribution provides multiple opportunities for
functional interactions between endogenous
cannabinoid substances, such as anandamide
(Devane et al., 1992; Di Marzo et al., 1994),
and ascending dopamine pathways.
-
- That these interactions may occur in vivo
isindicated by several observations. First, in
the striatum of freely moving rats, anandamide
release is greatly increased after activation of
dopamine D2 family receptors with the selective
agonist quinpirole (Giuffrida et al., 1999).
Second, pretreatment with theCB1 cannabinoid
antagonist SR141716A enhances the stimulation of
motor behavior elicited by systemic
administration ofquinpirole (Giuffrida et al.,
1999), although it has little effect perse on
basal motor activity (Rinaldi-Carmona et al.,
1994; Comptonet al., 1996; Navarro et al.,
1997). Third, injection of D2 family agonists
into basal ganglia nuclei opposes the behavioral
response to locally administered CB1 receptor
agonists (Sanudo-Pena etal., 1996, 1998;
Sanudo-Pena and Walker, 1998).
-
- Finally, chronic treatment with D2 family
antagonists results in upregulated expressionof
CB1 receptor mRNA in striatum (Mailleux and
Vanderhaeghen, 1993). Together, these findings
suggest that one of the functions of anandamide
in the CNS may be to modulate dopamine D2
receptor-induced facilitation of psychomotor
activity.In agreement with this possibility,
anandamide and other CB1agonists inhibit
movement, produce catalepsy, and
attenuated-amphetamine-induced hyperactivity and
stereotypy (Pryor etal., 1978; Gorriti et al.,
1999), whereas disruption of the CB1receptor
gene profoundly affects movement control (Ledent
etal., 1999; Zimmer et al., 1999).
-
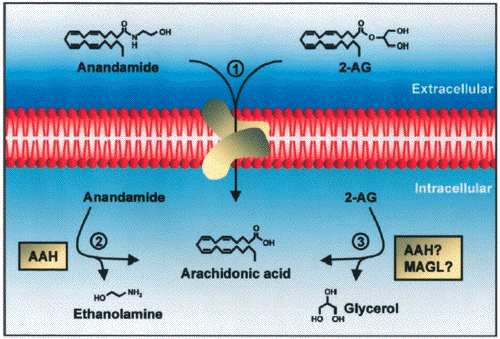
- When anandamide is administered as a drug,
its effects arecurtailed by a two-step mechanism
consisting of transport intocells, mediated by a
high-affinity carrier system (Beltramo et
al.,1997b; Hillard et al., 1997; Piomelli et
al., 1999), followed by intracellular
hydrolysis, catalyzed by a relatively
nonselective amidohydrolase enzyme (Deutsch and
Chin, 1993; Desarnaud et al., 1995; Cravatt et
al., 1996). Consequently, the anandamide
transport inhibitor
N-(4-hydroxyphenyl)-arachidonamide (AM404)
prolongs and enhances several responses to
exogenous anandamide,including analgesia
(Beltramo et al., 1997b) and vasodilatation
(Calignano et al., 1997a).
-
- We hypothesized that blockade of anandamide
transport, by causing this lipid to accumulate
at itssites of release, may help uncover a
participation of anandamide in the control of
dopamine neurotransmission and might offer a
pharmacological strategy to correct pathological
conditions characterized by dopaminergic
dysfunction. To test this hypothesis, we
investigated the pharmacological properties of
AM404 in the rat CNS and examined the effects of
this drug on behavioral responses elicited by
the activation of D2 family receptors.
[...]
 - DISCUSSION There is both experimental
and medical interest in developingmolecules that
selectively interfere with anandamide transport.
Anandamide transport inhibitors may be used
experimentally to uncover the functions of the
endocannabinoid system, which are still
essentially uncharacterized (for review, see
Piomelli et al.,1998). Furthermore,
anandamide transport inhibitors may offer a
rational approach to a variety of disease
conditions in which elevation of anandamide
levels at its release sites may result in a more
selective pharmacological response than direct
activation of CB1 receptors by agonist
drugs.
-
- In the present study, we used the anandamide
transport inhibitor AM404 to investigate
functional interactions between anandamide and
dopamine in the control of motor activity. The
existence of such interactions was suggested by
four key observations.
-
- First, in the striatum of freely moving
rats, activation of D2 family receptors
stimulates anandamide release (Giuffrida et
al.,1999). Second, blockade of CB1 cannabinoid
receptors enhances the stimulation of motor
behavior elicited by D2 agonists (Giuffridaet
al., 1999). Third, CB1 agonists and D2 family
agonists exert opposing behavioral effects when
they are administered by local injection into
individual basal ganglia nuclei
(San˜udo-Pena et al. 1996, 1998; SanudoPena
and Walker, 1998). Finally, treatment with D2
family antagonists causes an upregulation of
CB1receptor expression in striatum (Mailleux and
Vanderhaeghen,1993).
-
- In keeping with these results, we found that
AM404 counteracts two characteristic responses
mediated by activation of D2 family receptors:
apomorphine-induced yawning and quinpirole
induced stimulation of motor behaviors. These
effects are achieved at doses of AM404 that may
elicit only a mild hypokinesia when the drug is
administered alone and may selectively inhibit
anandamide transport in vitro.
-
- In addition, doses of AM404 identical to
those used in the present study are able to
produce a time-dependent increase in the levels
of anandamide in peripheral blood (A. Giuffrida,
F. Rodrġguez de Fonseca, F.Nava, J. Belluzzi,
and D. Piorrelli, in preparation). Thus, our
results are consistent with the hypothesis that
anandamide released by stimulation of D2 family
receptors participates in th econtrol of
dopamine-induced psychomotor
activation.
-
- CB1 receptor agonists elicit a broad
spectrum of behavioral responses that include
catalepsy, analgesia, reduced movement and
hypothermia (Pertwee, 1997). The finding that
AM404 evokes only a moderate slow-onset
hypokinesia when it is administered alone
demarcates the pharmacological profile of this
anandamide transport inhibitor from those of
direct-acting cannabimimetic drugs. This
distinction may result from the ability of AM404
to enhance anandamide signaling in an activity
dependent manner by causing anandamide to
accumulate in discrete regions of the CNS only
when release of this endocannabinoid substance
is triggered by appropriate stimuli.
-
- In the absence of such stimuli, tonic
anandamide release may be very low,
accountingfor the weak and slow-developing motor
effects of AM404 in naive animals. We considered
that the pharmacological profile of AM404 could
offer an original strategy to correct behavioral
abnormalities that are generally associated with
dysfunction in dopamine neurotransmission. As an
initial test of this hypothesis, we examined the
effects of AM404 in SHR, a rat line in which
hyperactivityand attention deficits have been
linked to a defective regulation of
mesocorticolimbic dopamine pathways (Esposito et
al.,1999; Russell, 2000; Sadile, 2000). We found
that administration of a low systemic dose of
AM404 (1 mg/kg) normalizes motoractivity in SHR
with no overt motor effect in WKY controls, the
strain from which SHR originate (Okamoto,
1969).
-
- These results suggest that
pharmacological inhibition of anandamide
inactivation may alleviate hyperactivity in
SHR. Additional experiments are needed to
determine whether this effect is mediated by an
elevation of anandamide levels in brain regions
involved in the control of movement and
attention.The multiple physiological functions
served by dopamine in the control of psychomotor
activity and the lack of animal models that
capture the complexities of psychiatric diseases
make it difficult to extrapolate from rodent
models to human syndromes.Yet, the spectrum of
pharmacological properties displayed by AM404
and the ability of this drug to counteract
potential manifestations of dopamine
dysregulation suggest that anandamide transport
may be a valuable target for the development of
novelneuropsychiatric medicines.
The
endogenous cannabinoid, anandamide, activates
the hypothalamo-pituitary-adrenal axis in CB1
cannabinoid receptor knockout
mice.
- Wenger
T, Ledent C, Tramu G
- Department of Human
Morphology and Developmental Embryology,
Semmelweis University, Budapest,
Hungary.
- Neuroendocrinology 2003; 78; 6; 294-300
The purpose of this study was to investigate
the effects of the endogenous cannabinoid
arachidonoyl-ethanolamide, anandamide (AEA), on
the activity of the
hypothalamo-pituitary-adrenal (HPA) axis in
cannabinoid receptor (CB(1) receptor)
inactivated (KO) mice. A low dose (0.01 mg/kg
i.p.) of AEA significantly increased plasma
corticotropin (ACTH) and corticosterone
concentrations in both wild-type (+/+) and in
mutant (-/-) animals. In each case, hormone
levels reached their peaks at 90 min after AEA
administration. In a parallel experiment, AEA
administration was preceded by the injection of
SR 141716A (1.0 mg/kg), a selective and potent
CB(1) receptor antagonist, or of capsazepine
(5.0 mg/kg), a potent vanilloid receptor of type
1 (VR1) antagonist. The latter drugs did not
prevent the effects of AEA on the HPA axis.
Using Fos protein immunohistochemistry, we
observed that the parvocellular part of the
hypothalamic paraventricular nucleus (PVN) was
activated as early as 45 min after AEA injection
and reached peak levels after 60 min in both +/+
and -/- mice. Furthermore, the CB(1) and VR1
receptor antagonists did not block the effects
of AEA on Fos immunoreactivity. The results
strongly support the view that activation of the
HPA axis produced by AEA possibly occurs via a
currently unknown (CB(x)) cannabinoid receptor
present in PVN.
|



